Abstract
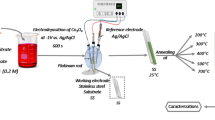
In this work, we have obtained nickel–cobalt oxide coatings by a simple two-step synthesis including electrochemical deposition and thermal treatment at 473 K, 573 K and 673 K in air atmosphere. Electrodeposition was carried out by varying the potential at the same time intervals and using one electrolyte bath containing nickel and cobalt salts. A glass tray with a fluorine-doped tin oxide coating was chosen as a chemically inert and high-temperature resistant substrate. Structural analysis by X-ray diffraction confirmed the formation of spinel-type nickel cobaltite NiCo2O4. The influence of annealing temperature on the pseudocapacitive performance of the films has been investigated using cyclic voltammetry and galvanostatic charge–discharge techniques in 0.1 M NaOH. NiCo2O4 nanostructures exhibited good electrochemical performance with high specific capacitance of 1332 F g−1 at 1 A g−1, and excellent cycle stability with 95% retention of SC after 500 charge–discharge cycles.









Similar content being viewed by others
References
N. Garg, M. Basu, A.K. Ganguli, J. Phys. Chem. C 118, 17332 (2014)
W. Cao, W. Wang, H. Shi et al., Nano Res. 11, 1437 (2018)
M. Cao, X. Wang, W. Cao et al., Small 14, 1800987 (2018)
D.P. Dubal, R. Holze, P. Gomez-Romero, Sci. Rep. 4, 7349 (2014)
P.S. Gaikar, S.T. Navale, S.L. Gaikwad et al., Dalton Trans. 46, 3393 (2017)
M.D. Stoller, R.S. Ruoff, Energy Environ. Sci. 3, 1294 (2010)
B. Conway, Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications, 1st edn. (Kluwer-Plenum, New York, 1999), pp. 221–257
G. Wang, L. Zhang, J. Zhang, Chem. Soc. Rev. 41, 797 (2012)
R. Kotz, M. Carlen, Electrochim. Acta 45, 2483 (2000)
E. Umeshbabu, G. Rajeshkhanna, G.R. Rao, Int. J. Hydrog. Energ. 39, 15627 (2014)
C. Yuan, H.B. Wu, Y. Xie et al., Angew. Chem. - Int. Ed. 53, 1488 (2014)
Z. Zhang, Q. Tan, Y. Chen et al., J. Mater. Chem. A 2, 5041 (2014)
V. Kumar, C.R. Mariappan, R. Azmi et al., ACS Omega 2, 6003 (2017)
F. Shi, X. Wang, C. Gu et al., RSC Adv. 4, 41910 (2014)
Y. Chen, H. Gan, J.H. Guan et al., J. Alloy. Compd. 760, 6 (2018)
T.H. Kim, G.K. Veerasubramani, S.J. Kim, J. Ind. Eng. Chem. 61, 181 (2018)
S. Liu, D. Ni, H.F. Li et al., J. Mater. Chem. A 6, 10674 (2018)
W.J. Zhou, D.D. Zhao, M.W. Xu et al., Electrochim. Acta 53, 7210 (2008)
G.H.A. Therese, P.V. Kamath, Chem. Mater. 12, 1195 (2000)
R.S. Jayashree, P. Vishnu Kamath, J. Power Sources 93, 273 (2001)
J. Yang, H. Liu, W.N. Martens et al., J. Phys. Chem. C 114, 111 (2010)
R.S. Jayashree, P. Vishnu Kamath, J. Appl. Electrochem. 29, 449 (1999)
R.S. Jayashree, P.V. Kamath, J. Mater. Chem. 9, 961 (1999)
D.S. Hall, D.J. Lockwood, C. Bock et al., Proc. R. Soc. A 471, 20140792 (2014)
Z. Zhang, Y. Jiang, X. Zheng et al., New J. Chem. 42, 11285 (2018)
Y. Gao, H. Li, G. Yang, Cryst. Growth Des. 15, 4475 (2015)
R.L. Doyle, M.E.G. Lyons, Phys. Chem. Chem. Phys. 15, 5224 (2013)
V. Gupta, T. Kusahara, H. Toyama et al., Electrochem. Commun. 9, 2315 (2007)
S. Shahrokhian, S. Rahimi, R. Mohammadi, Int. J. Hydrog. Energ. 43, 2256 (2018)
L. Jiang, Y. Sui, J. Qi et al., Appl. Surf. Sci. 426, 148 (2017)
B. Raveau, M. Seikh, Cobalt Oxides: From Crystal Chemistry to Physics, 1st edn. (Wiley-VCH Verlag GmbH & Co. KGaA, Germany, 2012), pp. 211–231
E. Umeshbabu, G. Rajeshkhanna, P. Justin et al., Mater. Chem. Phys. 165, 235 (2015)
Y.H. Chen, J.F. Zhou, D. Mullarkey et al., AIP Adv. 5, 087122 (2015)
Y. Si, C. Guo, C. Xie et al., Materials 11, 1912 (2018)
R.J. Deokate, R.S. Kalubarme, C.J. Park et al., Electrochim. Acta 224, 378 (2017)
F. Zhifu, W. Haihong, L. Zhikun et al., Vacuum 137, 125 (2017)
D. Li, F. Yu, Z. Yu et al., Mater. Lett. 158, 17 (2015)
V.G. Hadjiev, M.N. Iliev, I.V. Vergilov, J. Phys. C Solid State 21, L199 (1988)
Y. Li, W. Qiu, F. Qin et al., J. Phys. Chem. C 120, 4511 (2016)
X. He, X. Song, W. Qiao et al., J. Phys. Chem. C 119, 9550 (2015)
Y. Li, F.M. Li, X.Y. Meng et al., Nano Energy 54, 238 (2018)
A. Azor, M.L. Ruiz-Gonzalez, F. Gonell et al., Chem. Mater. 30, 4986 (2018)
Q. Liao, N. Li, S. Jin et al., ACS Nano 9, 5310 (2015)
W.K. Behl, J.E. Toni, J. Electroanal. Chem. 31, 63 (1971)
N. Maki, N. Tanaka, Cobalt, 1st edn. (Marcel Dekker Inc., USA, 1985), pp. 367–382
M. Jayalakshmi, M.M. Rao, K.B. Kim, Int. J. Electrochem. Sci. 1, 324 (2006)
G. Periyasamy, K. Annamalai, Appl. Surf. Sci. 449, 705 (2018)
P. Taberna, P. Simon, Electrochemical Techniques, 1st edn. (Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2013), pp. 111–130
R.C. Ambare, B.J. Lokhande, J. Mater. Sci. -Mater. El. 29, 16289 (2018)
L. Halder, A. Maitra, A.K. Das et al., Electrochim. Acta 283, 438 (2018)
C. Lamiel, Y.R. Lee, M.H. Cho et al., J. Colloid Interf. Sci. 507, 300 (2017)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Barauskienė, I., Valatka, E. Layer-by-layer electrodeposition of high-capacitance nickel–cobalt oxides on FTO substrate. J Mater Sci: Mater Electron 30, 10311–10320 (2019). https://doi.org/10.1007/s10854-019-01369-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-019-01369-y