Abstract
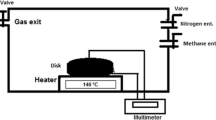
Synthesis of magnesium doped zinc oxide nanostructures has been carried out by vapor transport method and characterization of the samples have been performed regarding methane (CH4) gas sensing in addition to their structural, morphological, chemical composition and optical properties. The un-doped and magnesium doped zinc oxide nanostructures were synthesized on silicon substrates at 900 °C through vapor liquid solid mechanism. Powder X-ray diffraction study confirmed the growth of material exhibiting crystalline wurtzite (hexagonal) structure. Scanning electron microscopy revealed that morphology of grown material is in the form of nanorods and nanobelts with average diameter and thickness of 12.66 ± 3.72 µm and 1.88 ± 0.70 µm, respectively. Energy dispersive X-ray analysis was used to examine the stoichiometry of the samples. Optical characterizations were carried out by photoluminescence, diffused reflectance spectroscopy, voltage dependent photo-current response and Time dependent photocurrent response. A significant change in energy bandgap has been observed after Mg incorporation in ZnO. For doped ZnO samples, the observed value of band gap is 3.32 eV which is higher than that for undoped ZnO (3.18 eV).The nanostructures were tested for UV and gas sensing properties based on the change in resistance in UV light when exposed to CH4 gas. The gas sensing response was recorded for temperature ranging from 50 to 200 °C for 400 ppm concentration of methane gas. The sensing response of Mg-doped ZnO nanobelts was found as high as 54%. The Mg-doped ZnO nanobelts showed significant, stable and enhanced sensing properties (54%) towards 400 ppm of CH4 gas at optimal temperature of 200 °C. The observations revealed that Mg doping in ZnO nanostructures would help to improve the CH4 and UV sensing of these materials.






Similar content being viewed by others
References
Q. Qi et al., Humidity sensing properties of KCl-doped ZnO nanofibers with super-rapid response and recovery. Sens. Actuators B 137(2), 649–655 (2009)
P. Ivanov et al., Development of high sensitivity ethanol gas sensors based on Pt-doped SnO2 surfaces. Sens. Actuators B 99(2), 201–206 (2004)
R. Vander Wal et al., Metal-oxide nanostructure and gas-sensing performance. Sens. Actuators B 138(1), 113–119 (2009)
F. Röck, N. Barsan, U. Weimar, Electronic nose: current status and future trends. Chem. Rev. 108(2), 705–725 (2008)
X. Zhou et al., Humidity detection by nanostructured ZnO: a wireless quartz crystal microbalance investigation. Sens. Actuators A 135(1), 209–214 (2007)
H.-K. Hong et al., Portable electronic nose system with gas sensor array and artificial neural network. Sens. Actuators B 66(1), 49–52 (2000)
M. Madkour, Y.K. Abdel-Monem, F. Al, Sagheer, Controlled synthesis of NiO and Co3O4 nanoparticles from different coordinated precursors: impact of precursor’s geometry on the nanoparticles characteristics. Ind. Eng. Chem. Res. 55(50), 12733–12741 (2016)
A. Gedanken, Y. Mastai, Sonochemistry and other novel methods developed for the synthesis of nanoparticles. Chem. Nanomater. 10, 113–169 (2005)
S.T. Tan et al., Blueshift of optical band gap in ZnO thin films grown by metal-organic chemical-vapor deposition. J. Appl. Phys. 98(1), 013505 (2005)
S.N. Das et al., ZnO single nanowire-based UV detectors. Appl. Phys. Lett. 97(2), 022103 (2010)
B. Mondal et al., Zinc oxide nano-platelets for effective methane gas-sensing applications. Chin. J. Phys. 51(5), 994–1005 (2013)
M. Amin et al., Effects of Mg doping on optical and CO gas sensing properties of sensitive ZnO nanobelts. CrystEngComm 16(27), 6080–6088 (2014)
L.-J. Bie et al., Nanopillar ZnO gas sensor for hydrogen and ethanol. Sens. Actuators B 126(2), 604–608 (2007)
M. Kumar et al., Pd/ZnO nanorods based sensor for highly selective detection of extremely low concentration hydrogen. Sci. Rep. 7(1), 236 (2017)
B. Lei et al., Tuning electronic properties of In2O3 nanowires by doping control. Appl. Phys. A 79(3), 439–442 (2004)
Y.K. Abdel-Monem, S.M. Emam, H.M. Okda, Solid state thermal decomposition synthesis of CuO nanoparticles from coordinated pyrazolopyridine as novel precursors. J. Mater. Sci. 28(3), 2923–2934 (2017)
R. Maity et al., Synthesis and characterization of ZnO nano/microfibers thin films by catalyst free solution route. Phys. E 25(4), 605–612 (2005)
H.M. Xiong et al., Sonochemical synthesis of highly luminescent zinc oxide nanoparticles doped with magnesium (II). Angew. Chem. Int. Ed. 48(15), 2727–2731 (2009)
J. Singh et al., Synthesis, band-gap tuning, structural and optical investigations of Mg doped ZnO nanowires. CrystEngComm 14(18), 5898–5904 (2012)
N. Wang, Y. Cai, R. Zhang, Growth of nanowires. Mater. Sci. Eng. 60(1), 1–51 (2008)
H.J. Fan et al., Vapour-transport-deposition growth of ZnO nanostructures: switch between c-axial wires and a-axial belts by indium doping. Nanotechnology 17(11), S231 (2006)
M. Hafeez et al., Catalyst solubility and self-doping in ZnS nanostructures. J. Appl. Phys. 111(2), 024313 (2012)
J.-H. Park et al., Ultrawide ZnO nanosheets. J. Mater. Chem. 14(1), 35–36 (2004)
Z.L. Wang, X. Kong, J. Zuo, Induced growth of asymmetric nanocantilever arrays on polar surfaces. Phys. Rev. Lett. 91(18), 185502 (2003)
H. Pan et al., Electroluminescence and field emission of Mg-doped ZnO tetrapods. Nanotechnology 17(20), 5096 (2006)
Y. Zhang et al., Symmetric and asymmetric growth of ZnO hierarchical nanostructures: nanocombs and their optical properties. Nanotechnology 17(8), 1916 (2006)
Y.K. Abdel-Monem, Efficient nanophotocatalyt of hydrothermally synthesized anatase TiO2 nanoparticles from its analogue metal coordinated precursor. J. Mater. Sci. 27(6), 5723–5728 (2016)
A.K. Zak et al., Starch-stabilized synthesis of ZnO nanopowders at low temperature and optical properties study. Adv. Powder Technol. 24(3), 618–624 (2013)
M. Nagasawa, S. Shionoya, Second class exciton structure in stannic oxide. J. Phys. Soc. Jpn. 30(1), 158–167 (1971)
E. Burstein, Anomalous optical absorption limit in InSb. Phys. Rev. 93(3), 632 (1954)
Q. Dong-Jiang et al., Characterizations of cubic ZnMgO films grown on Si (111) at low substrate temperature. Chin. Phys. Lett. 20(4), 582 (2003)
K.-S. Ahn et al., Enhanced photoelectrochemical responses of ZnO films through Ga and N codoping. Appl. Phys. Lett. 91(23), 231909 (2007)
J.L. Musfeldt, Handbook of applied solid state spectroscopy edited by DR Vij (Kurukshetra University, India). J. Am. Chem. Soc. 129(10), 3028–3028
H.-C. Hsu et al., Band gap engineering and stimulated emission of ZnMgO nanowires. Appl. Phys. Lett. 89(1), 013101 (2006)
U. Manzoor, D.K. Kim, Synthesis and enhancement of ultraviolet emission by post-thermal treatment of unique zinc oxide comb-shaped dendritic nanostructures. Script. Mater. 54(5), 807–811 (2006)
Y. Zhang et al., Brush-like hierarchical ZnO nanostructures: synthesis, photoluminescence and gas sensor properties. J. Phys. Chem. C 113(9), 3430–3435 (2009)
A.A. Ibrahim et al., Growth and properties of Ag-doped ZnO nanoflowers for highly sensitive phenyl hydrazine chemical sensor application. Talanta 93, 257–263 (2012)
N. Han et al., Evaluating the doping effect of Fe, Ti and Sn on gas sensing property of ZnO. Sens. Actuators B 147(2), 525–530 (2010)
G. Dar et al., Ce-doped ZnO nanorods for the detection of hazardous chemical. Sens. Actuators B 173, 72–78 (2012)
Y. Wang et al., Mesostructured SnO2 as sensing material for gas sensors. Solid State Elect. 48(5), 627–632 (2004)
W.-F. Chung et al., Environment-dependent thermal instability of sol-gel derived amorphous indium-gallium-zinc-oxide thin film transistors. Appl. Phys. Lett. 98(15), 152109 (2011)
Acknowledgements
Higher education commission (HEC) Pakistan is acknowledged for financial support through Project No. 9294/NRPU/R&D/HEC/2017. The authors would also be thankful to COMSATS University Islamabad for necessary funds through project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
GuL, M., Amin, M., Abbas, M. et al. Synthesis and characterization of magnesium doped ZnO nanostructures: methane (CH4) detection. J Mater Sci: Mater Electron 30, 5257–5265 (2019). https://doi.org/10.1007/s10854-019-00825-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-019-00825-z