Abstract
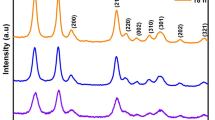
Tin oxide (SnO2) nanoparticles were synthesized employing simple sol–gel method. Modification in the structural, morphological and optical properties of the as-synthesized tin oxide nanoparticles due to various solution pH (6–12) and thermal annealing at 400 °C (Experiment 1) was studied. X-ray diffraction results of the tin oxide nanoparticles prepared from the precursor solution pH 8 and annealed at 400 °C showed the formation of tin oxide tetragonal phase (SnO2-t) and the surface morphology of the SnO2-t nanoparticles studied by scanning electron microscope revealed the formation of spherical shaped agglomerations. Hence, the tin oxide nanoparticles prepared from the solution pH 8 were annealed at 200, 400, 600 and 800 °C in order to study the effect of annealing at various temperatures on the structural, morphological, optical and vibrational properties of tin oxide nanoparticles (Experiment 2). When the annealing temperature was increased to 600 and 800 °C, mixed phases of SnO2-t and tin oxide orthorhombic system (SnO-o) were formed. Various solution pH and annealing temperatures influenced the direct band gap value. SnO2-t phase synthesized from the solution pH 8 and annealed at 400 °C showed a direct band gap of ~4.50 eV. The tin oxide samples annealed at various temperatures showed a slight shift in the fluorescence peak observed at ~327 nm. Raman studies of the samples obtained from Experiment 1 and Experiment 2 showed a slight shift in the vibrational frequency. I–V studies carried out to investigate the electrical properties of the SnO2 thin film formed by simple drop casting method revealed better ohmic contact and its suitability for gas sensing applications.







Similar content being viewed by others
References
P. Chetri, A. Choudhury, E. Physica, Low-Dimens. Syst. Nanostruct. 47, 257–263 (2013)
D.L. Feldheim, C.A. Foss, Metal Nanoparticles: Synthesis, Characterization, and Applications (Taylor & Francis, New York, 2001), pp. 7874–7875
L. Aswaghosh, D. Manoharan, N.V. Jaya, Phys. Chem. Chem. Phys. 8, 1–11 (2016)
R. Adan, N.A. Razana, I.A. Rahman, M.A. Farrukh, J. Chin. Chem. Soc. 57, 222–229 (2010)
M. Batzhill, U. Diebold, Prog. Surf. Sci. 79, 47–154 (2005)
L. Cojocaru, C. Olivier, T. Toupance, E. Sellierb, L. Hirsch, J. Mater. Chem. A 44, 13789–13799 (2013)
M.M. Rashad, I.A. Ibrahim, I. Osama, A.E. Shalan, Bull. Mater. Sci. 37, 903–909 (2014)
A.K. Singh, U.T. Nakate, Adv. Nanopart. 2, 66–70 (2013)
S.H. Ng, D.I.D. Santos, S.Y. Chew, D. Wexler, J. Wang, S.X. Dou, H.K. Liu, Electrochem. Commun. 9, 915–919 (2007)
Q. Tian, Y. Tian, Z. Zhang, L. Yang, S.I. Hirano, Power Sour. 269, 479–485 (2014)
H. Xiguang, M. Jin, S. Xie, Q. Kuang, Z. Jiang, Y. Jiang, Z. Xie, L. Zheng, Angew. Chem. Int. Ed. 48, 9180–9183 (2009)
L. Li, S. Chen, L. Xu, Y. Bai, Z. Nie, H. Liu, L. Qi, Mater. Chem. B 2, 1121–1124 (2014)
A.S. Reddy, N.M. Figueiredo, A. Cavaleiro, J. Phys. Chem. Solids 74, 825–829 (2003)
C.M. Liu, X.T. Zu, Q.M. Wei, L.M. Wang, J. Phys. D 39, 2494–2497 (2006)
Y. Cheng, R. Yang, J.P. Zheng, Z.L. Wang, P. Xiong, Mater. Chem. Phys. 137, 372–380 (2012)
Z.R. Dai, Z.W. Pan, Z.L. Wang, Solid State Commun. 118, 351–354 (2001)
S.M. Priya, A. Geetha, K. Ramamurthi, J Sol-Gel Sci. Technol. 78, 365–372 (2016)
D. Chen, L. Gao, Chem. Phys. Lett. 398, 201–206 (2004)
H. Yang, Y. Hu, A. Tang, S. Jin, G. Qiu, J. Alloys Compd. 363, 271–274 (2004)
G.E. Patil, D.D. Kajale, V.B. Gaikwad, G.H. Jain, Int. Nano Lett. 2, 17 (2012)
N. Rajesh, J.C. Kannan, T. Krishnakumar, S.G. Leonardi, G. Neri, Sens. Actuator B-Chem. 194, 96–104 (2014)
E.T.H. Tan, G.W. Ho, A.S.W. Wong, S. Kawi, A.T.S. Wee, Nanotechnology 19, 255706 + 07 (2008)
S. Gnanam, V. Rajendran, J. Sol–Gel Sci. Technol. 53, 555–559 (2010)
H. Giefers, F. Porsch, G. Wortmann, Solid State Ionics 176, 199–207 (2005)
M. Meyer, G. Onida, A. Ponchel, L. Reining, Comp. Mater. Sci. 10, 319–324 (1998)
K. Nose, A.Y. Suzuki, N. Oda, M. Kamiko, Y. Mitsuda, Appl. Phys. Lett. 104(1–4), 091905 (2014)
C.D. Gu, H. Zheng, X.L. Wang, J.P. Tu, RSC Adv. 5, 9143–9153 (2015)
D. Hu, B. Han, S. Deng, Z. Feng, Y. Wang, J. Popovic, M. Nuskol, Y. Wang, I. Djerdj, J. Phys. Chem. C 118, 9832–9840 (2014)
A. Diallo, E. Manikandan, V. Rajendran, M. Maaza, J. Alloys Compd. 681, 561–570 (2016)
K. Lokesh, G. Kavitha, E. Manikandan, G.K. Mani, K. Kaviyarasu, J.B.B. Rayappan, R. Ladchumananandasivam, J.S. Aanand, M. Jayachandran, M. Maaza, IEEE 16, 2477–2483 (2016)
P.K. Sharma, V.V. Varadan, V.K. Varadan, J. Eur. Ceram. Soc. 23, 659–666 (2003)
B. Houng, C.L. Huang, S.Y. Tsai, J. Cryst. Growth 307, 328–333 (2007)
N. Shanmugam, S. Cholan, N. Kannadasan, N. Sathishkumar, N. Viruthagiri, J. Nanomater. 2013, 1–7 (2013)
L.L. Hench, J.K. West, Chem. Rev. 90, 33–72 (1990)
M. Marikannan, V. Vishnukanthan, A. Vijayashankar, J. Mayandi, J.M. Pearce, AIP Adv. 5(1-), 027122 (2015). -027122–8
Z. Wang, S. Liu, T. Jiang, X. Xu, .J Zhang, C. An, C. Wang, RSC Adv. 5, 64582–64587 (2015)
B.D. Cullity, S.R. Stock, Elements of X-ray Diffraction, 3rd edn. (Prentice Hall, New York, 2001), pp. 78–103
B. Sathyaseelan, E. Manikandan, V. Lakshmanan, I. Bashkar, K. Sivakumar, R. Ladchumananandasivam, J. Kennedy, M. Maaza, J. Alloys Compd. 671, 486–492 (2016)
A. Muthukumar, D. Arivuoli, E. Manikandan, M. Jayachandran, Opt. Mater. 47, 88–94 (2015)
R. Wahab, Y.S. Kim, H.S. Shin, Mater. Trans. 50, 2092–2097 (2009)
R. Krahne, L. Manna, G. Morello, A. Figureolo, C. George, S. Deka, Physical Properties of Nanorods (Nanoscience and Technology, New York, 2013)
V. Agrahari, A.K. Tripathi, M.C. Mathpal, A.C. Pandey, S.K. Mishra, R.K. Shukla, A. Agarwal, J. Mater. Sci. 26, 9571–9582 (2015)
M. Saravanakumar, S. Agilan, N. Muthukumaraswamy, A. Marusamy, K. Prabakaran, A. Ranjitha, U.P. Maheshwari, Indian J. Phys. 88, 831–835 (2014)
M. Pherson, A. Richard, R.P. Matthew, Henry’s clinicAl Diagnosis and Management by Laboratory Methods E Book, 23rd edn. (Elsevier Health Sciences, Missouri, 2017), pp. 1–1568
J. Tauc, Liquid Amorphous, Semiconductors, (Springer Science & Business Media, New York, 2012), pp. 159–220
T. Shukla, J. Sens. Technol. 2, 102–108 (2012)
O. Lupan, L. Chow, G. Chai, A. Schulte, S. Park, H. Heinrich, Mater. Sci. Eng.-B 157, 101–104 (2009)
X. Mathew, J.P. Enriquez, C.M. García, G.C. Puente, M.A.C. Jacome, J.A.T. Antonio, J. Hays, A. Punnoose, J. Appl. Phys. 100, 0739071–0739077 (2006)
S.H. Sun, G.W. Meng, G.X. Zhang, T. Gao, B.Y. Geng, L.D. Zhang, J. Zuo, Chem. Phys. Lett. 376, 103–107 (2003)
M.N. Rumyantseva, A.M. Gaskov, N. Rosman, T. Pagnier, J.R. Morante, Chem. Mater. 17, 893–901 (2005)
J. Geurts, S. Rau, W. Richter, F.J. Schmitte, Thin Solid Films 121, 217–225 (1984)
M. Poloju, N. Jayababu, E. Manikandan, M.V.R. Reddy, J. Mater. Chem. C 5, 2662–2668 (2017)
S.S. Pan, C. Ye, X.M. Teng, L. Li, G.H. Li, Appl. Phys. Lett. 89(1–3), 251911 (2006)
K. Anandan, V. Rajendiran, J. Phys. Sci. 19, 129–141 (2014)
A. Azam, A.S. Ahmed, S.S. Habib, A.H. Naqvi, J. Alloys Compd. 523, 83–87 (2012)
R.K. Mishra, A. Kushwaha, P.P. Sahay, RSC Adv. 4, 3904–3912 (2014)
A. Johari, M.C. Bhatnagar, V. Rana, Adv. Mat. Lett. 3, 515–518 (2012)
J.HuangY. Liu, Y. Wu, X. Li, Am. J. Analyt. Chem. 8, 60–71 (2017)
O. Teresa, Trans. Electr. Electron. Mater. 18, 21–24 (2017)
D.R. Viji, N. Singh, Luminescence and Related Properties of II-VI Semiconductors (Nova Publishers, New York, 1998), pp. 99–101
Acknowledgements
One of the authors (MP) sincerely thanks SRM University, Chennai, for the award of SRM research fellowship to carry out the work. The authors gratefully acknowledge Prof. D. John Thiruvadigal, Dean of Science and humanities, Dr. Preferencial Kala, Head, department of Physics and Nanotechnology and SRM University for extending the facilities created under (DST-FIST SR/FST/PSI-155/2010). The authors also thank Centre for Nanoscience and Nanotechnology and to Nanotechnology Research Centre, SRM University, for extending the facilities.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Subramaniam, M.P., Arunachalam, G., Kandasamy, R. et al. Effect of pH and annealing temperature on the properties of tin oxide nanoparticles prepared by sol–gel method. J Mater Sci: Mater Electron 29, 658–666 (2018). https://doi.org/10.1007/s10854-017-7959-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-017-7959-2