Abstract
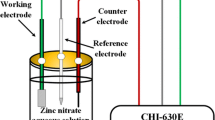
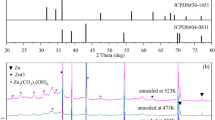
ZnS thin films were prepared by chemical bath codeposition using ZnSO4–ZnCl2 or Zn(CH3COO)2–ZnCl2 as zinc ion sources. The presence of SO4 2− favors the heterogeneous growth of ZnS thin film. The coexistence of two zinc salts impedes the formation of homogeneous precipitation and improves the growth rate of ZnS film. XRD and HRTEM results show that all the samples exhibit the cubic structure. EDS analysis shows that Zn/S atom ratios from the codeposition are closer to 1:1 than those deposited from a single zinc salt, and ZnS thin films of S3 and S7 are very uniform without stirring. FTIR reveals that –NH2 group as a surface modifier is adsorbed on the surface of ZnS nanoparticles. Raman spectra further reveal that S3, S4 and S7 form the ZnS films, and ZnO phase is present in short or middle range of the S6 nanocrystal, indicating that different amounts of zinc salts affect the structure of ZnS films significantly after three 2.5 h deposition cycles. The grain sizes determined by FESEM are inversely proportional to RMS determined by AFM. The band gap values of ZnS thin films agree well with the results of HRTEM. The photocurrent responses of different samples are similar, indicating that different amounts of zinc salts have little effect on the photocurrent of ZnS films. The photocatalytic performance of S6 and S8 is much better than that of S1–S5. S6 decomposes 65 % of methyl orange within 3 h, and its K value is 4.78 × 10−1 h−1. The photocatalytic performance is induced by the growth mechanism, which determines the grain size of ZnS thin film. The tendency of grain sizes of ZnS films agrees well with that of photocatalytic performance, especially under the clusters by clusters deposition.














Similar content being viewed by others
References
F. Lu, W.P. Cai, Y.G. Zhang, Y. Li, F.Q. Sun, Fabrication and field-emission performance of zinc sulfide nanobelt arrays. J. Phys. Chem. C 111, 13385–13392 (2007)
K. Manzoor, S.R. Vadera, N. Kumar, T.R.N. Kutty, Synthesis and photoluminescent properties of ZnS nanocrystals doped with copper and halogen. Mater. Chem. Phys. 82, 718–725 (2003)
Y. Zhang, X.Y. Dang, J. Jin, T. Yu, B.Z. Li, Q. He, F.Y. Li, Y. Sun, ZnS thin film deposited with chemical bath deposition process directed by different stirring speeds. Appl. Surf. Sci. 256, 6871–6875 (2010)
Y. Liao, F. Yu, L. Long, B. Wei, L. Lu, J. Zhang, Low-cost and reliable thin film encapsulation for organic light emitting diodes using magnesium fluoride and zinc sulfide. Thin Solid Films 519, 2344–2348 (2011)
W. Daranfed, M.S. Aida, A. Hafdallah, H. Lekiket, Substrate temperature influence on ZnS thin films prepared by ultrasonic spray. Thin Solid Films 518(4), 1082–1084 (2009)
P. Roy, J.R. Ota, S.K. Srivastava, Crystalline ZnS thin films by chemical bath deposition method and its characterization. Thin Solid Films 515, 1912–1917 (2006)
C.M. Huang, L.C. Chen, G.T. Pan, T.C.K. Yang, W.S. Chang, K.W. Cheng, Effect of Ni on the growth and photoelectrochemical properties of ZnS thin films. Mater. Chem. Phys. 117, 156–162 (2009)
S.M.B. Ghorashi, A. Behjat, M. Neghabi, G. Mirjalili, Effects of air annealing on the optical, electrical, and structural properties of nanostructured ZnS/Au/ZnS films. Appl. Surf. Sci. 257, 1602–1606 (2010)
X. Fang, T. Zhai, U.K. Gautam, L. Li, L. Wu, Y. Bando, D. Golberg, ZnS nanostructures: from synthesis to applications. Prog. Mater Sci. 56, 175–287 (2011)
X.S. Fang, Y. Bando, M.Y. Liao, U.K. Gautam, C.Y. Zhi, B. Dierre, B.D. Liu, T.Y. Zhai, T. Sekiguchi, Y. Koide, D. Golberg, Single-crystalline ZnS nanobelts as ultraviolet-light sensors. Adv. Mater. 21, 2034–2039 (2009)
X.S. Fang, Y. Bando, M.Y. Liao, T.Y. Zhai, U.K. Gautam, L. Li, Y. Koide, D. Golberg, An efficient way to assemble ZnS nanobelts as ultraviolet-light sensors with enhanced photocurrent and stability. Adv. Funct. Mater. 20, 500–508 (2010)
M. Stefan, E.J. Popovici, O. Pana, E. Indrea, Synthesis of luminescent zinc sulphide thin films by chemical bath deposition. J. Alloys Compd. 548, 166–172 (2013)
J.M. Doña, J. Herrero, Process and film characterization of chemical-bath-deposited ZnS thin film. J. Electrochem. Soc. 141, 205–210 (1994)
S. Siebentritt, Alternative buffers for chalcopyrite solar cells. Sol. Energy 77, 767–775 (2004)
U. Gangopadhyay, K. Kim, D. Mangalaraj, J. Yi, Low cost CBD ZnS antireflection coating on large area commercial mono-crystalline silicon solar cells. Appl. Surf. Sci. 230, 364–370 (2004)
J. Cheng, D.B. Fan, H. Wang, B.W. Liu, Y.C. Zhang, H. Yan, Chemical bath deposition of crystalline ZnS thin films. Semicond. Sci. Technol. 18, 676–679 (2003)
R.S. Mane, C.D. Lokhande, Chemical deposition method for metal chalcogenide thin films. Mater. Chem. Phys. 65, 1–31 (2000)
E.J. Ibanga, C.L. Luyer, J. Mugnier, Zinc oxide waveguide produced by thermal oxidation of chemical bath deposited zinc sulphide thin films. Mater. Chem. Phys. 80, 490–495 (2003)
J. Britt, C. Ferekides, Thin-film CdS/CdTe solar cell with 15.8% efficiency. Appl. Phys. Lett. 62, 2851–2852 (1993)
J.M. Doña, J. Herrero, Chemical bath codeposited CdS-ZnS film characterization. Thin Solid Films 268, 5–12 (1995)
J. Vidal, O. Vigil, O. de Melo, N. Lopez, O.Z. Angel, Influence of NH3 concentration and annealing in the properties of chemical bath deposited ZnS films. Mater. Chem. Phys. 61, 139–142 (1999)
Q. Liu, G.B. Mao, J.P. Ao, Chemical bath-deposited ZnS thin films: preparation and characterization. Appl. Surf. Sci. 254, 5711–5714 (2008)
B. Asenjo, A.M. Chaparro, M.T. Gutiérrez, J. Herrero, J. Klaer, Study of CuInS2/ZnS/ZnO solar cells, with chemically deposited ZnS buffer layers from acidic solutions. Sol. Energy Mater. Sol. Cells 92, 302–306 (2008)
H. Ke, S.W. Duo, T.Z. Liu, Q. Sun, C.X. Ruan, X.Y. Fei, J.L. Tan, S. Zhan, Effect of temperature on structural and optical properties of ZnS thin films by chemical bath deposition without stirring the reaction bath. Mater. Sci. Semicond. Process. 18, 28–35 (2014)
A.X. Wei, J. Liu, M.X. Zhuang, Y. Zhao, Preparation and characterization of ZnS thin films prepared by chemical bath deposition. Mater. Sci. Semicond. Process. 16, 1478–1484 (2013)
T.B. Nasr, N. Kamoun, C. Guasch, Physical properties of ZnS thin films prepared by chemical bath deposition. Appl. Surf. Sci. 254, 5039–5043 (2008)
W. Vallejo, M. Hurtado, G. Gordillo, Kinetic study on Zn(O, OH)S thin films deposited by chemical bath deposition. Electrochim. Acta 55, 5610–5616 (2010)
W. Vallejo, C. Quińones, G. Gordillo, A comparative study of thin films of Zn(O; OH)S and In(O; OH)S deposited on CuInS2 by chemical bath deposition method. J. Phys. Chem. Solids 73, 573–578 (2012)
A. Antony, K.V. Murali, R. Manoj, M.K. Jayaraj, The effect of the pH value on the growth and properties of chemical-bath-deposited ZnS thin films. Mater. Chem. Phys. 90, 106–110 (2005)
P.A. Luque, C.M.G. Gutiérrez, G. Lastra, A.C. Castillo, M.A.Q. López, A. Olivas, Role of zinc source in chemical bath deposition of zinc sulfide thin films on Si3N4. J. Electron. Mater. 43, 4317–4321 (2014)
M. Cao, B.L. Zhang, L. Li, J. Huang, S.R. Zhao, H. Cao, J.C. Jiang, Y. Sun, Y. Shen, Effects of zinc salts on the structural and optical properties of acidic chemical bath deposited ZnS thin films. Mater. Res. Bull. 48, 357–361 (2013)
T.Z. Liu, H. Ke, H. Zhang, S.W. Duo, Q. Sun, X.Y. Fei, G.Y. Zhou, H. Liu, L.J. Fan, Effect of four different zinc salts and annealing treatment on growth, structural, mechanical and optical properties of nanocrystalline ZnS thin films by chemical bath deposition. Mater. Sci. Semicond. Process. 26, 301–311 (2014)
T.Z. Liu, Y.Y. Li, H. Ke, Y.H. Qian, S.W. Duo, Y.L. Hong, X.Y. Sun, Chemical bath co-deposited ZnS film prepared from different zinc salts: ZnSO4–Zn(CH3COO)2, Zn(NO3)2–Zn(CH3COO)2, and ZnSO4–Zn(NO3)2. J. Mater. Sci. Technol. 32, 207–217 (2016)
K. Kočí, L. Matějová, O. Kozák, L. Čapek, V. Valeš, M. Reli, P. Praus, K. Safářová, A. Kotarba, L. Obalová, ZnS/MMT nanocomposites: the effect of ZnS loading in MMT on the photocatalytic reduction of carbon dioxide. Appl. Catal. B Environ. 158–159, 410–417 (2014)
X.J. Xu, L.F. Hu, N. Gao, S.X. Liu, S. Wageh, A.A.A. Ghamdi, A. Alshahrie, X.S. Fang, Controlled growth from ZnS nanoparticles to ZnS–CdS nanoparticle hybrids with enhanced photoactivity. Adv. Funct. Mater. 25, 445–454 (2015)
C. Lu, C.Z. Liu, R. Chen, X.X. Fang, K. Xu, D.W. Meng, Synthesis and characterization of ZnO/ZnS/CuS ternary nanocomposites as high efficient photocatalyst in visible light. J. Mater. Sci. Mater. Electron. 27, 6947–6954 (2016)
Y. Tian, G.F. Huang, L.J. Tang, M.G. Xia, W.Q. Huang, Z.L. Ma, Size-controllable synthesis and enhanced photocatalytic activity of porous ZnS nanospheres. Mater. Lett. 83, 104–107 (2012)
Y. Chen, G.F. Huang, W.Q. Huang, L.L. Wang, Y. Tian, Z.L. Ma, Z.M. Yang, Annealing effects on photocatalytic activity of ZnS films prepared by chemical bath deposition. Mater. Lett. 75, 221–224 (2012)
Y. Chen, G.F. Huang, W.Q. Huang, B.S. Zou, A. Pan, Enhanced visible-light photoactivity of La-doped ZnS thin films. Appl. phys. A Mater. 108, 895–900 (2012)
Y.F. Chai, G.F. Huang, L.L. Wang, W.Q. Huang, J. Zhou, Enhanced photocatalytic activity and stability of ZnxCd1−xS/TiO2 nanocomposites synthesized by chemical bath deposition. Mater. Lett. 142, 133–136 (2015)
S.D. Sartale, B.R. Sankapal, M.L. Steiner, A. Ennaoui, Preparation of nanocrystalline ZnS by a new chemical bath deposition route. Thin Solid Films 480–481, 168–172 (2005)
J.A. Dean, Lange’s Handbook of Chemistry, 13th edn. (McGraw-Hill Book Company, New York, 1987), p. 5
P. Roy, S.K. Srivastava, A new approach towards the growth of cadmium sulphide thin film by CBD method and its characterization. Mater. Chem. Phys. 95, 235–241 (2006)
D.A. Johnston, M.H. Carletto, K.T.R. Reddy, I. Forbes, R.W. Miles, Chemical bath deposition of zinc sulfide based buffer layers using low toxicity materials. Thin Solid Films 403–404, 102–106 (2002)
D. Lincot, R.O. Borges, Chemical Bath deposition of cadmium sulfide thin films. In situ growth and structural studies by combined quartz crystal microbalance and electrochemical impedance techniques. J. Electrochem. Soc. 139, 1880–1889 (1992)
W.L. Liu, C.S. Yang, S.H. Hsieh, W.J. Chen, C.L. Fern, Effect of deposition variables on properties of CBD ZnS thin films prepared in chemical bath of ZnSO4/SC(NH2)2/Na3C3H5O7/NH4OH. Appl. Surf. Sci. 264, 213–218 (2013)
A.K. Kole, S. Gupta, P. Kumbhakar, P.C. Ramamurthy, Nonlinear optical second harmonic generation in ZnS quantum dots and observation on optical properties of ZnS/PMMA nanocomposites. Opt. Commun. 313, 231–237 (2014)
G. Murugadoss, Synthesis, optical, structural and thermal characterization of Mn2+ doped ZnS nanoparticles using reverse micelle method. J. Lumin. 131, 2216–2223 (2011)
T.A. Safeera, N. Johns, E.I. Anila, A.I. Martinez, P.V. Sreenivasan, R. Reshmi, M. Sudhanshu, M.K. Jayaraj, Low temperature fabrication and characterization of wurtzite structured ZnS quantum dots by chemical spray pyrolysis. J. Anal. Appl. Pyrol. 115, 96–102 (2015)
X.B. Zhang, H.W. Song, L.X. Yu, T. Wang, X.G. Ren, X.G. Kong, Y.H. Xie, X.J. Wang, Surface states and its influence on luminescence in ZnS nanocrystallite. J. Lumin. 118, 251–256 (2006)
S.N. Azizi, M.J. Chaichi, P. Shakeri, A. Bekhradnia, Determination of atropine using Mn-doped ZnS quantum dots as novel luminescent sensitizers. J. Lumin. 144, 34–40 (2013)
L.J. Feng, C.Y. Wang, Z.L. Ma, C.L. Lü, 8-Hydroxyquinoline functionalized ZnS nanoparticles capped with amine groups: a fluorescent nanosensor for the facile and sensitive detection of TNT through fluorescence resonance energy transfer. Dyes Pigments 97, 84–91 (2013)
M. Pal, N.R. Mathews, E.R. Morales, J.M.G. Jiménez, X. Mathew, Synthesis of Eu+3 doped ZnS nanoparticles by a wet chemical route and its characterization. Opt. Mater. 35, 2664–2669 (2013)
D.A. Reddy, C.L. Liu, R.P. Vijayalakshmi, B.K. Reddy, Effect of Al doping on the structural, optical and photoluminescence properties of ZnS nanoparticles. J. Alloys Compd. 582, 257–264 (2014)
J. Serrano, A. Cantarero, M. Cardona, N. Garro, R. Lauck, R.E. Tallman, T.M. Ritter, B.A. Weinstein, Raman scattering in β-ZnS. Phys. Rev. B 69, 014301 (2004)
S. Kumar, C.L. Chen, C.L. Dong, Y.K. Ho, J.F. Lee, T.S. Chan, R. Thangavel, T.K. Chen, B.H. Mok, S.M. Rao, M.K. Wu, Room temperature ferromagnetism in Ni doped ZnS nanoparticles. J. Alloys Compd. 554, 357–362 (2013)
M. Hossu, R.O. Schaeffer, L. Ma, W. Chen, Y.B. Zhu, R. Sammynaiken, A.G. Joly, On the luminescence enhancement of Mn2+ by co-doping of Eu2+ in ZnS: Mn, Eu. Opt. Mater. 35, 1513–1519 (2013)
R. Cuscó, E.A. Lladó, J. Ibáñez, L. Artús, Temperature dependence of Raman scattering in ZnO. Phys. Rev. B 75, 165202 (2007)
H.X. Li, M.X. Xia, G.Z. Dai, H.C. Yu, Q.L. Zhang, A.L. Pan, T.H. Wang, Y.G. Wang, B.S. Zou, Growth of oriented zinc oxide nanowire array into novel hierarchical structures in aqueous solutions. J. Phys. Chem. C 112, 17546–17553 (2008)
S. Kumar, P.D. Sahare, Observation of band gap and surface defects of ZnO nanoparticles synthesized via hydrothermal route at different reaction temperature. Opt. Commun. 285, 5210–5216 (2012)
X.S. Fang, L.M. Wu, L.F. Hu, ZnS nanostructure arrays: a developing material star. Adv. Mater. 23, 585–598 (2011)
Y. Lei, F.F. Chen, R. Li, J. Xu, A facile solvothermal method to produce graphene–ZnS composites for superior photoelectric applications. Appl. Surf. Sci. 308, 206–210 (2014)
L.H. Yu, H. Ruan, Y. Zheng, D.Z. Li, A facile solvothermal method to produce ZnS quantum dots-decorated graphene nanosheets with superior photoactivity. Nanotechnology 24, 375601 (2013)
M. Sookhakian, Y.M. Amin, R. Zakaria, W.J. Basirun, M.R. Mahmoudian, B.N. Tabrizi, S. Baradaran, M. Azarang, Significantly improved photocurrent response of ZnS-reduced graphene oxide composites. J. Alloys Compd. 632, 201–207 (2015)
L.H. Yu, W. Chen, D.Z. Li, J.B. Wang, Y. Shao, M. He, P. Wang, X.Z. Zheng, Inhibition of photocorrosion and photoactivity enhancement for ZnO via specific hollow ZnO core/ZnS shell structure. Appl. Catal. B Environ. 164, 453–461 (2015)
S.H. Xu, L. Fu, T. Song, H. Pham, A. Yu, F.G. Han, L. Chen, Preparation of ZnO flower/reduced graphene oxide composite with enhanced photocatalytic performance under sunlight. Ceram. Int. 41, 4007–4013 (2015)
W.Q. Peng, G.V. Cong, S.C. Qu, Z.G. Wang, Synthesis and photoluminescence of ZnS: Cu nanoparticles. Opt. Mater. 29, 313–317 (2006)
S.S. Kumar, M.A. Khadar, K.G.M. Nair, Analysis of the effect of annealing on the photoluminescence spectra of Cu+ ion implanted ZnS nanoparticles. J. Lumin. 131, 786–789 (2011)
S.R. Chalana, R. Vinodkumar, I. Navas, V. Ganesan, V.P.M. Pillai, Influence of argon ambience on the structural, morphological and optical properties of pulsed laser ablated zinc sulfide thin films. J. Lumin. 132, 944–952 (2012)
T.R. Giraldi, G.V.F. Santos, V.R. Mendonca, C. Ribeiro, I.T. Weber, Annealing effects on the photocatalytic activity of ZnO nanoparticles. J. Nanosci. Nanotechnol. 11, 3635–3640 (2011)
Acknowledgments
The authors would like to appreciate the financial supports of National Natural Science Foundation of China (No. 51363007), Natural Science Foundation of Jiangxi Province (No. 20132BAB206033), Foundation of Jiangxi Educational Commission (Nos. GJJ14588 and KJLD13070), Project of Jiangxi Youth Scientist (No. 20122BCB23031), and Science Foundation of Jiangxi Science and Technology Normal University (Nos. 2014QNBJRC005, 2015CXTD003 and 3000990328).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Li, Y., Liu, Z., Duo, S. et al. Structural, optical, photocurrent and mechanism-induced photocatalytic properties of surface-modified ZnS thin films by chemical bath deposition. J Mater Sci: Mater Electron 28, 28–42 (2017). https://doi.org/10.1007/s10854-016-5489-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-016-5489-y