Abstract
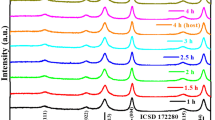
Nanostructured manganese doped magnesium aluminate (Mg1−xMnxAl2O4, where x = 0.0, 0.25, 0.50, 0.75, 1.0) were prepared by a sol–gel method. All the samples were characterised by TGA, XRD, SEM, EDAX, TEM and IR spectroscopy techniques. The semiconducting nature of materials were investigated by DC resistivity measurement. The TGA curves show that spinel the oxides are formed at 600 °C. The XRD studies reveal formation of cubic spinel phase with average crystallite size of 28 nm. The composition of Mg1−xMnxAl2O4, (x = 0.0, 0.50, 1.0) shows spherical interlinked fibrous morphology. The elemental compositions determined by energy dispersive X-ray analysis (EDAX) indicate desired composition. Particle size obtained from TEM analysis was found to be ~23 nm. The IR spectra show two strong characteristic absorption bands at tetrahedral and octahedral sites. The temperature dependent variation of dc resistivity of material reflects semiconducting nature of materials.







Similar content being viewed by others
References
A. Goldstein, J. Eur. Ceram. Soc. 32, 2869 (2012)
F.S. Al-Hazmi, W.E. Mahmoud, J. Eur. Ceram. Soc. 34(12), 3047 (2014)
B. Ismail, S.T. Hussain, S. Akram, Chem. Eng. J. 219, 395 (2013)
A.E. Lavat, M.C. Grasselli, E.G. Lovecchio, Ceram. Int. 36, 15 (2010)
N.M. Khalil, M.B. Hassan, E.M.M. Ewais, F.A. Saleh, J. Alloys Compd. 496, 600 (2010)
A. Laobuthee, S. Wongkasemjit, E. Traversa, J. Eur. Ceram. Soc. 20, 91 (2000)
M.J. Iqbal, B. Ismail, C. Rentenberger, H. Ipser, Mater. Res. Bull. 46, 2271 (2011)
T. Shiono, K. Shiono, K. Miyamoto, G. Pezzotti, J. Am. Ceram. Soc. 83, 235 (2000)
J. Guo, H. Lou, H. Zhao, X. Wang, X. Zheng, Mater. Lett. 58, 1920 (2004)
R. Jiang, Z. Xie, C. Zhang, Q. Chen, Catal. Today 93–95, 359 (2004)
M.A.L. Braulio, M. Rigaud, A. Buhr, C. Parr, V.C. Pandolfelli, Ceram. Int. 37(6), 1705 (2011)
F.R. Perez, C.A. Barrero, A.R.H. Walker, K.E. García, K. Nomura, Mater. Chem. Phys. 117(1), 214 (2009)
B.Q. Zhu, B.X. Fang, X.C. Li, Ceram. Int. 36, 2493 (2010)
B.Q. Zhu, B.X. Fang, X.C. Li, X. Jiang, J. Chin. Ceram. Soc. 38, 730 (2010)
A. Rahman, R. Jayaganthan, J. Nanostruct. Chem. 3, 147 (2015)
M.F. Zawrah, H. Hamaad, S. Meky, Ceram. Int. 33, 969 (2007)
R.B. Jotania, P.A. Patel, Int. J. Eng. Res. Appl. 2, 494 (2012)
Y. Suyama, A. Kato, Ceram. Int. 8, 17 (1982)
C.T. Wang, L.S. Lin, S.J. Yang, J. Am. Ceram. Soc. 75, 2240 (1992)
A. Jouini, A. Yoshikawaa, T. Fukudaa, G. Boulonb, J. Cryst. Growth 293, 517 (2006)
V.T. Gritsyna, YuG Kazarinov, V.B. Kol’ner, L.A. Lytvynov, K.E. Sickafus, Funct. Mater. 12, 719 (2005)
P.P. Hankare, V.T. Vader, U.B. Sankpal, R.P. Patil, A.V. Jadhav, I.S. Mulla, J. Mater. Sci. Mater. Electron. 22, 1109 (2011)
A.S. Tapase, R.P. Patil, S.D. Delekar, I.S. Mulla, P.P. Hankare, J. Mater. Sci. Mater. Electron. 25, 369 (2014)
P.V.M. Kutty, S. Dasgupta, Ceram. Int. 39, 7891 (2013)
S.H. Seok, S.H. Choi, E.D. Park, S.H. Han, J.S. Lee, J. Catal. 209, 6 (2002)
W.M. Shaheen, K.S. Hong, Thermochim. Acta 381, 153 (2002)
A. Saberi, F. Golestani-Fard, H. Sarpoolaky, M. Willert-Porada, T. Gerdes, R. Simon, J. Alloys Compd. 462, 142 (2008)
K.J. Standley, Oxide Magnetic Materials (Clarendon press, Oxford, 1962)
A. Cottrell, An Introduction to Metallurgy (Edward Arnold, London, 1967)
Acknowledgments
Author (PPH) is very thankful to UGC, New Delhi for financial assistance through UGC-BSR fuculty fellowship F. No. 18-1(46)/2013 (BSR). ISM is grateful to CSIR India for granting him Emeritus Scientist scheme.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mali, A.V., Wandre, T.M., Sanadi, K.R. et al. Synthesis, characterization and electrical properties of novel Mn substituted MgAl2O4 synthesized by sol–gel method. J Mater Sci: Mater Electron 27, 613–619 (2016). https://doi.org/10.1007/s10854-015-3796-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-015-3796-3