Abstract
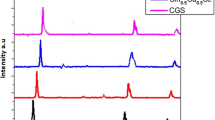
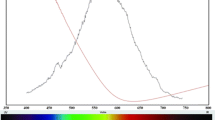
CuInS2 nanoparticles as the visible (wurtzite, 1.67 eV) or near infrared (chalcopyrite, 1.50 eV) light absorbing material in thin film solar cells, were synthesized using facile, one step heating up method by dissolving of CuCl, InCl3 and SC(NH2)2 as precursors in oleylamine (OLA) alone or in combination with oleic acid (OA) and 1-octadecene (ODE) as solvent. The phase, size, morphology, and size distribution were controlled by the coordination ability between solvent molecules and metal precursors, reaction temperature and time. The presence of higher amounts of thiourea or OA to OLA led to the formation of chalcopyrite phase in comparison to wurtzite structure. Also, higher reaction temperatures (>240 °C) resulted in favour of more chalcopyrite phase and higher crystallinity but the nanoparticles got agglomerated. As synthesized nanoparticles was characterized by transmission electron microscopy, X-ray diffraction, X-ray photoelectron spectroscopy, high resolution-transmission electronic microscopy, ultraviolet–visible-near infrared, photoluminescence. The high resolution TEM confirmed the existence of chalcopyrite structure along with wurtzite structure in the nanocrystal (polytypism). Well controlled chalcopyrite CuInS2 triangular pyramidal shape with an average size ranging from ~10–20 nm size was obtained by using 20 ml OLA or 20 ml OLA along with 4 ml OA and ODE, respectively, with 210 °C heating up and 4 h annealing time.











Similar content being viewed by others
References
M.H. Valdés, M. Berruet, A. Goossens, M. Vázquez, Spray deposition of CuInS2 on electrodeposited ZnO for low-cost solar cells. Surf. Coat. Tech. 204, 3995–4000 (2010)
A. Aboulaich, M. Michalska, R. Schneider, A. Potdevin, J. Deschamps, R. Deloncle, G. Chadeyron, R. Mahiou, Ce-doped YAG nanophosphor and red emitting CuInS2/ZnS core/shell quantum dots for warm white light-emitting diode with high color rendering index. ACS Appl. Mater. Interfaces. 6, 252–258 (2014)
S. Tomic, L. Bernasconi, B.G. Searle, N.M. Harrison, Electronic and optical structure of wurtzite CuInS2. J. Phys. Chem. C 118, 14478–14484 (2014)
K.T. Yong, I. Roy, R. Hu, H. Ding, H. Cai, J. Zhu, X. Zhang, E.J. Bergeya, P.N. Prasad, Synthesis of ternary CuInS2/ZnS quantum dot bioconjugates and their applications for targeted cancer bioimaging. Integr. Biol. 2, 121–129 (2010)
Y. Chen, S. Li, L. Huang, D. Pan, Green and facile synthesis of water-soluble Cu–In–S/ZnS core/shell quantum dots. Inorg. Chem. 52, 7819–7821 (2013)
H. Yoon, S.H. Na, J.Y. Choi, M.W. Kim, H. Kim, H.S. An, B.K. Min, S.J. Ahn, J.H. Yun, J. Gwak, K.H. Yoon, S.S. Kolekar, M.F.A.M.V. Hest, S.S. Al-Deyab, M.T. Swihart, S.S. Yoon, Carbon- and oxygen-free Cu(InGa)(SSe)2 solar cell with a 4.63% conversion efficiency by electrostatic spray deposition. ACS Appl. Mater. Interfaces 6, 8369–8377 (2014)
D. Aldakov, A. Lefrancois, P. Reiss, Ternary and quaternary metal chalcogenide nanocrystals: synthesis, properties and applications. J. Mater. Chem. C 1, 3756–3776 (2013)
J. Kolny-Olesiak, H. Weller, Synthesis and application of colloidal CuInS2 semiconductor nanocrystals. ACS Appl. Mater. Interfaces. 5, 12221–12237 (2013)
N. Bao, X. Qiu, Y.H.A. Wang, Z. Zhou, X. Lu, C.A. Grimes, A. Gupta, Facile Thermolysis synthesis of CuInS2 nanocrystals with tunable anisotropic shape and structure. Chem. Commun. 47, 9441–9443 (2011)
M. Gusain, P. Kumar, R. Nagarajan, Wurtzite CuInS2: solution based one pot direct synthesis and its doping studies with non-magnetic Ga3+ and magnetic Fe3+ ions. RSC Adv. 3, 18863–18871 (2013)
F.B. Dejene, The structural and material properties of CuInSe2 and Cu(In, Ga)Se2 prepared by selenization of stacks of metal and compound precursors by Se vapor for solar cell applications. Sol. Energy Mater. Sol. Cells 93, 577–582 (2009)
P.S. Vasekar, A.H. Jahagirdar, N.G. Dhere, Photovoltaic characterization of copper-indium-gallium sulfide (CIGS2) solar cells for lower absorber thicknesses. Thin Solid Films 518, 1788–1790 (2010)
T. Koehler, S. Gledhill, A. Grimm, N. Allsop, C. Camus, A. Hänsel, W. Bohne, J. Röhrich, M. Lux-Steiner, C.H. Fischer, An Investigation of the dip-ion layer gas reaction process to produce ZnO films with increased deposition rates. Thin Solid Films 517, 3332–3339 (2009)
T. Todorov, J. Carda, P. Escribano, A. Grimm, J. Klaer, R. Klenk, Electro deposited In2S3 buffer layers for CuInS2 solar cells. Sol. Energy Mater. Sol. Cells 92, 1274–1278 (2008)
R. Sharma, S. Shim, R.S. Mane, T. Ganesh, A. Ghule, G. Cai, D.H. Ham, S.K. Min, W. Lee, S.H. Han, Optimization of growth of ternary CuInS2 thin films by ionic reactions in alkaline chemical bath as n-Type photoabsorber layer. Mater. Chem. Phys. 116, 28–33 (2009)
S.M. Hosseinpour-Mashkani, F. Mohandes, M. Salavati-Niasari, K. Venkateswara-Rao, Microwave-assisted synthesis and photovoltaic measurements of CuInS2 nanoparticles prepared by using metal-organic precursors. Mater. Res. Bull. 47, 3148–3159 (2012)
D.Y. Lee, J.H. Kim, Deposition of CuInS2 films by electrostatic field assisted ultrasonic spray pyrolysis. Sol. Energy Mater. Sol. Cells 95, 245–249 (2011)
B. Koo, R.N. Patel, B.A. Korgel, Wurtzite-chalcopyrite polytypism in CuInS2 nanodisks. Chem. Mater. 21, 1962–1966 (2009)
Y. Vahidshad, A. Irajizad, R. Ghasemzadeh, S.M. Mirkazem, A. Masoud, Structural and optical characterization of nanocrystalline CuAlS2 chalcopyrite synthesized by polyol method in autoclave. Int. J. Mod. Phys. B 26(1250179), 1–12 (2012)
Y. Vahidshad, R. Ghasemzadeh, A. Irajizad, S.M. Mirkazemi, Synthesis and characterization of copper indium sulfide chalcopyrite structure with hot injection method. J. Nanostruct. 3, 145–154 (2013)
J. Guo, W.H. Zhou, M. Li, Z.L. Hou, J. Jiao, Z.J. Zhou, S.X. Wu, Synthesis of bullet-like wurtzite CuInS2 nanocrystals under atmospheric conditions. J. Cryst. Growth 359, 72–76 (2012)
D.H. Jara, S.J. Yoon, K.G. Stamplecoskie, P.V. Kamat, Size-dependent photovoltaic performance of CuInS2 quantum dot-sensitized solar cells. Chem. Mater. 26, 7221–7228 (2014)
Y. Vahidshad, M.N. Tahir, A. Irajizad, S.M. Mirkazemi, R. Ghasemzadeh, W. Tremel, Structural and optical properties of Fe and Zn substitution in CuInS2 nanoparticles synthesized by a one-pot facile method. J. Mater. Chem. C. 3, 889–898 (2015)
W. Du, X. Qian, J. Yin, Q. Gong, Shape- and phase-controlled synthesis of monodisperse, single-crystalline ternary chalcogenide colloids through a convenient solution synthesis strategy. Chem. Eur. J. 13, 8840–8846 (2007)
Y. Vahidshad, R. Ghasemzadeh, A. Irajizad, S.M. Mirkazemi, A. Masoud, Solvothermal synthesis of CuMS2 (M = Al, In, Fe) nanoparticles and effect of coordinating solvent on the crystalline structur. Scientia Iranica. Trans. Nanotechol. 21, 2468–2478 (2014)
L. Shi, C. Pei, Q. Li, Ordered arrays of shape tunable CuInS2 nanostructures, from nanotubes to nano test tubes and nanowires. Nanoscale. 2, 2126–2130 (2010)
D. Pan, L. An, Z. Sun, W. Hou, Y. Yang, Z. Yang, Y. Lu, Synthesis of Cu–In–S ternary nanocrystals with tunable structure and composition. J. Am. Chem. Soc. 130, 5620–5621 (2008)
W.-C. Huang, C.-H. Tseng, S.-H. Chang, H.-Y. Tuan, C.-C. Chiang, L.-M. Lyu, M.H. Huang, Solvothermal synthesis of zincblende and wurtzite CuInS2 nanocrystals and their photovoltaic application. Langmuir 28, 8496–8501 (2012)
R.G. Pearson, Hard and soft acids and bases, HSAB, Part 1: fundamental principles. J. Chem. Educ. 45, 581–587 (1968)
M. Kruszynska, H. Borchert, J. Parisi, J. Kolny-Olesiak, Synthesis and shape control of CuInS2 nanoparticles. J. Am. Chem. Soc. 132, 15976–15986 (2010)
S.T. Connor, C.M. Hsu, B.D. Weil, S. Aloni, Y. Cui, Phase transformation of biphasic Cu2S − CuInS2 to monophasic CuInS2 nanorods. J. Am. Chem. Soc. 131, 4962–4966 (2009)
Y. Qi, Q. Liu, K. Tang, Z. Liang, Z. Ren, X. Liu, Synthesis and characterization of nanostructured wurtzite CuInS2: a new cation disordered polymorph of CuInS2. J. Phys. Chem. C 113, 3939–3944 (2009)
W. Yue, S. Han, R. Peng, W. Shen, H. Geng, F. Wu, S. Tao, M. Wang, CuInS2 quantum dots synthesized by a solvothermal route and their application as effective electron acceptors for hybrid solar cells. J. Mater. Chem. 20, 7570–7578 (2010)
S.T. Connor, B.D. Weil, S. Misra, Y. Cui, M.F. Toney, Behaviors of Fe, Zn, and Ga substitution in CuInS2 nanoparticles probed with anomalous X-ray diffraction. Chem. Mater. 25, 320–325 (2013)
F. Gong, S. Tian, B. Liu, D. Xiong, X. Zhao, Oleic acid assisted formation mechanism of CuInS2 nanocrystals with tunable structures. RSC Adv. 4, 36875–36881 (2014)
T. Yamamoto, I.V. Luck, R. Scheer, H.K. Yoshida, Differences in the electronic structure and compensation mechanism between n-Type Zn- and Cd-doped CuInS2 crystals. Phys. B 273–274, 927–929 (1999)
J. Chang, E.R. Waclawik, Controlled synthesis of CuInS2, Cu2SnS3 and Cu2ZnSnS4 nanostructures: insight into the universal phase-selectivity mechanism. Cryst. Eng. Commun. 15, 5612–5619 (2013)
G. Will, E. Hinze, A. Rahman, M. Abdelrahman, Crystal structure analysis and refinement of digenite, Cu1.8S, in the temperature range 20 to 500 °C under controlled sulfur partial pressure. Eur. J. Mineral. 14, 591–598 (2002)
T. Kuzuya, Y. Hamanaka, K. Itoh, T. Kino, K. Sumiyama, Y. Fukunaka, S. Hirai, Phase control and its mechanism of CuInS2 nanoparticles. J. Colloid Interface Sci. 388, 137–143 (2012)
M.B. Sigman Jr, A. Ghezelbash, T. Hanrath, A.E. Saunders, F. Lee, B.A. Korgel, Solventless synthesis of monodisperse Cu2S nanorods, nanodisks, and nanoplatelets. J. Am. Chem. Soc. 125, 16050–16057 (2003)
J.-J. Wang, J.-S. Hu, Y.-G. Guo, L.I.-J. Wan, Wurtzite Cu2ZnSnSe4 nanocrystalsfor high-performance organic-inorganic hybrid photodetectors. NPG Asia Mater. 4, 1–10 (2012)
S. Kumar, T. Nann, Shape control of II–VI semiconductor nanomaterials. Small 2, 316–329 (2006)
E. Witt, J. Kolny-Olesiak, Recent developments in colloidal synthesis of CuInSe2 nanoparticles. Chem. Eur. J. 19, 9746–9753 (2013)
D.V. Talapin, J.-S. Lee, M.V. Kovalenko, E.V. Shevchenko, Prospects of colloidal nanocrystals for electronic and optoelectronic applications. Chem. Rev. 110, 389–458 (2010)
E.V. Shevchenko, D.V. Talapin, H. Schnablegger, A. Kornowski, O. Festin, P. Svedlindh, M. Haase, H. Weller, Study of nucleation and growth in the organometallic synthesis of magnetic alloy nanocrystals: the role of nucleation rate in size control of CoPt3 nanocrystals. J. Am. Chem. Soc. 125, 9090–9101 (2003)
J. Chang, E.R. Waclawik, Colloidal semiconductor nanocrystals: controlled synthesis and surface chemistry in organic media. RSC Adv. 4, 23505–23527 (2014)
Z.L. Wang, Transmission electron microscopy of shape-controlled nanocrystals and their assemblies. J. Phys. Chem. B. 104, 1153–1175 (2000)
Y. Xia, Y. Xiong, B. Lim, S.E. Skrabalak, Shape-Controlled synthesis of metal nanocrystals: simple chemistry meets complex physics? Angew. Chem. Int. Ed. 48, 60–103 (2009)
Z. Liu, L. Wang, Q. Hao, D. Wang, K. Tang, M. Zuo, Q. Yang, Facile synthesis and characterization of CuInS2 nanocrystals with different structures and shapes. Cryst. Eng. Comm. 15, 7192–7198 (2013)
X. Lu, Z. Zhuang, Q. Peng, Y. Li, Controlled synthesis of wurtzite CuInS2 nanocrystals and their side-by-side nanorod assemblies. Cryst. Eng. Comm. 13, 4039–4045 (2011)
T. Mokari, M. Zhang, P. Yang, Shape, size, and assembly control of Pbte nanocrystals. J. Am. Chem. Soc. 129, 9864–9865 (2007)
A.R. Tao, S. Habas, P. Yang, shape control of colloidal metal nanocrystals. Small 4, 310–325 (2008)
Y.W. Jun, J.S. Choi, J. Cheon, Shape control of semiconductor and metal oxide nanocrystals through nonhydrolytic colloidal routes. Angew. Chem. Int. Ed. 45, 3414–3439 (2006)
W.-J. Wang, Y. Jiangn, X.-Z. Lan, C. Wang, X.-M. Liu, B.-B. Wang, J.-W. Li, B. Yang, X.-N. Ding, Synthesis of CuInSe2 monodisperse nanoparticles and the nanorings shape evolution via a green solution reaction route. Mater. Sci. Semicond. Process. 15, 467–471 (2012)
H. Zhong, Y. Li, M. Ye, Z. Zhu, Y. Zhou, C. Yang, Y. Li, A facile route to synthesize chalcopyrite CuInSe2 nanocrystals in non-coordinating solvent. Nanotechnology 18(025602), 1–6 (2007)
S.R. Kodigala, V.S. Raja, A.K. Bhatnagar, F. Juang, S.J. Chang, Y.K. Su, Effect of annealing and γ-irradiation on the properties of CuInSe2 thin films. Mater. Lett. 45, 251–261 (2000)
K. Zeaiter, Y. Llinares, C. Llinares, Structural and photoluminescence study of the quaternary alloys system CuIn(SxSe1−x)2. Sol. Energy Mater. Sol. Cells 61, 313–329 (2000)
M.V. Yakushev, A.V. Mudryi, I.V. Victorov, J. Krustok, E. Mellikov, Energy of excitons in CuInS2 single crystals. Appl. Phys. Lett. 88(011922), 1–10 (2006)
J. Hofhuis, J. Schoonman, A. Goossens, Elucidation of the excited-state dynamics in CuInS2 thin films. J. Phys. Chem. C 112, 15052–15059 (2008)
Acknowledgments
This research was defined and supported by from the Iran university of Science and Technology. This research was supported by Professor Wolfgang Tremel at Johannes Gutenberg-University of Mainz.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vahidshad, Y., Tahir, M.N., Mirkazemi, S.M. et al. One-pot thermolysis synthesis of CuInS2 nanoparticles with chalcopyrite-wurtzite polytypism structure. J Mater Sci: Mater Electron 26, 8960–8972 (2015). https://doi.org/10.1007/s10854-015-3579-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-015-3579-x