Abstract
The effect of the substitutional and vacancy type defects on the H2 adsorption energy over a monolayer hexagonal boron nitride (h-BN) substrate has been studied by using the van der Waals density functional theory calculations. Carbon doping at the boron site or formation of boron vacancy can be an effective way to increase the adsorption energy value of a pristine h-BN substrate. The repulsive lateral interaction present in between the two H2 molecules plays a vital role in case of multiple H2 molecule adsorption over the substrate. Also, the carbon cluster formation during doping can have a favorable effect in the overall storage capacity of the h-BN substrate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since, last few decades, hydrogen (H2) is being considered as a promising alternative to the fossil fuels. But, the scarcity of an appropriate H2 storage material is one of the obstacles for its wide applications [1, 2]. While the conventional storage mechanisms involve the use of low temperature and high pressure, researchers have also considered the three-dimensional structures, such as metal–organic frameworks, metal hydrides and aerogels [1,2,3].
After the experimental synthesis of graphene in 2004 [4], two-dimensional materials are in the center of research attention. The monolayer materials like graphene [5], borophene [6], silicine [7], MoS2 [8], graphitic ZnO [9, 10], graphitic CN [11] and hexagonal boron nitride (h-BN) [12] were also investigated for the adsorption of H2 molecules. In 2009, Topsakal et al. theoretically established the stability of a two-dimensional honeycomb BN structure [13]. The nearest BN bonds are a combination of B-sp2 and N-sp2 orbitals, but, gain an ionic character due to the charge transfer between the B and N atoms [13]. After that, there were plenty of theoretical studies exploring the structural [13,14,15,16,17,18,19], electronic [13,14,15,16,17,18,19], optical [19], vibrational [20], magnetic [21] and thermoelectric [22] properties of both pristine and defect-induced h-BN monolayer. The monolayer and multilayer h-BN sheet was also prepared experimentally by different groups [23,24,25,26,27]. With large surface area and unique bonding character, h-BN also drew attention as a suitable candidate for the adsorption of different atmospheric and toxic gas molecules, e.g., N2 [28], H2O [29], H2 [30], CO [30, 31], HCl [30], CO2 [31, 32], H2S [31,32,33], HF [31], NO [31], SO2 [32, 33], SOF2 [33], SO2F2 [33], etc. In 2017, Fu et al. predicted the H2 storage capacity of a pure h-BN system with the help of a new equation of state [34]. Chettri et al. studied H2 adsorption over a h-BN monolayer with the help of density functional theory [12]. Moreover, bilayer h-BN was constructed for this purpose with [35] and without [36] an externally applied electric field. Other than the pristine structure, doped h-BN sheets were also considered in this context [37,38,39,40,41,42]. Transition metal ion-doped h-BN sheets were studied by Thupsuri et al. [37] and Shelvin et al. [38] for H2 adsorption. Zhou et al. [39] explored Ni, and Venkataramanan et al. [40] explored Ni and Rh doped h-BN sheets in this regard. Rare-earth metal, such as cerium decorated two-dimensional h-BN, was studied by Das et al. in 2020 [41]. In 2021, Naqvi et al. investigated the effect of C, Si and Ge doping over the adsorption of H2 molecules in a h-BN sheet [42]. In 2017, the adsorption of atomic H2 over h-BN sheets was considered by Hao et al. [43]. Porous BN [44] and BN nanotubes [45, 46] were considered by some research groups.
In the present work, the adsorption of H2 molecules is studied over the h-BN monolayers with incorporation of vacancy and substitutional carbon (C) defects. The effect of such vacancy and substitutional defects on the electrical and mechanical properties of monolayer h-BN was studied by Sagar et al. in 2014 [47]. Recently, Huang et al. studied the microscopic mechanism of vacancy and C substitutional defects in hexagonal h-BN in details using density functional theory (DFT) [48]. Experimentally, electron beam induced C doping in h-BN nanosheets was achieved by Wei et al. in 2011 [49]. C annealed h-BN has been prepared by several other research groups [50, 51]. In 2007, Shelvin et al. studied the H2 adsorption over defective h-BN sheets and nanotubes [46]. In 2014, Loh et al. employed an electric field to study the selective binding of H, O, H2 and O2 over a C-doped h-BN nanomesh using DFT calculations [52]. In 2016, Gao et al. observed the adsorption of O2 molecules over C-doped h-BN sheets [53]. The carbon-doped h-BN as a catalyst for NO reduction was considered by Mudchimo et al., was considered in 2018 [54]. In 2023, Mondinos et al. investigated the reaction of mono-atoms like H, Li, C, O, Al, Si, P and S with single vacancy induced h-BN monolayers [55].
The present work focuses on the variation of the H2 adsorption energy over the defect site, neighboring atomic sites and intermediate locations inside a defective monolayer h-BN sheet. The presence of lateral interaction between the two H2 molecules is taken into account to correctly predict the maximum packing of adsorbents over the substrate. Also, the carbon cluster formation during the doping can affect the value of adsorption energy.
Methodology
All the DFT calculations required for this work are performed using the MedeA VASP (Vienna ab initio simulation package) software package [56,57,58,59]. For structural optimization of the BN unit cell, H2 molecule and Bader charge analysis, GGA-PBE exchange correlation functional is used [60,61,62]. The adsorption energy of the H2 molecule over h-BN sheet is calculated using revPBE-vdW functional within the van der Waals DFT (vdW-DFT) framework [63]. A projector augmented wave (PAW) pseudopotential approximation, plane wave basis set with cutoff energy 400 eV and a 4 × 4 × 1 Monkhorst–Pack k-points grid are chosen for all the calculations [64]. The convergence criteria are set to be a maximum change of 10–5 eV in energy and 0.01 eV/Å in Hellman–Feynman force.
Results and discussion
Adsorption of a single H2 molecule over a pristine h-BN system
At the beginning, a unit cell of h-BN with one boron (B) and one nitrogen (N) atom is taken and fully relaxed using the GGA-PBE exchange correlation functional. The optimized bond length and lattice parameters (a = b) are obtained to be 1.45 and 2.51 Å, respectively. Our calculated values of bond length and lattice parameter are closely matching with the previously calculated values by Topsakal et al. [13]. A H2 molecule with GGA-PBE optimized bond length of 0.75 Å is also taken. Next, a 5 × 5 × 1 supercell containing twenty-five number of B and twenty-five number of N atoms has been constructed from the unit cell and it is structurally optimized by using the revPBE-vdW functional. A vacuum height of 16 Å is maintained to avoid interaction between two consecutive layers. To calculate the adsorption energy over a pristine h-BN substrate, the H2 molecule is placed at 6 Å above the B, N site and center position separately. The vertical gap, \(d\), between the substrate and the H2 molecule is decreased gradually, starting from 6 Å (\({d}_{\infty }\)). Hence, 6 Å and more vertical gap configuration (as depicted in Fig. 1a) is taken to be an isolated configuration with an energy value, \(E\left({d}_{\infty }\right)\) which is defined as \({E}_{\text{isolated}}\).
a A H2 molecule placed at 6 Å above a monolayer h-BN substrate. The green, gray and white spheres indicate the B, N and H atoms, respectively; Interaction energy (\({E}_{\text{interaction}}\)) curve for H2 adsorption over different sites of b pristine, c defect-induced h-BN system using revPBE-vdW functional.
In the isolated configuration, there is no interaction present between the substrate and the adsorbent. With every step size, the energy value of the system (\(E(d)\)) is calculated using revPBE-vdW functional. It is to be mentioned that, although the bond length and orientation of the H2 molecule has been relaxed during each of the energy calculation, the substrate dynamics are ignored. In this manuscript, the interaction energy \({E}_{interaction}\) is defined as the difference between \(E(d)\) and \({E}_{\text{isolated}}\).
The interaction energy versus distance curve for the placement of the H2 molecule over B and N site is depicted in Fig. 1b. The curves in Fig. 1b are van der Waals type in nature and have a minimum at a distance, say \({d}_{0}\), with an energy, say \({E}_{\text{adsorption}}\). \({E}_{\text{adsorption}}\) is the maximum attractive adsorption energy lying in the physisorption regime.
The adsorption energy (height) calculated above the B, N site and the center location for a pristine 5 × 5 × 1 h-BN supercell is − 64.7 meV (at 3.0 Å), − 66.2 meV (at 3.0 Å) and − 66.8 meV (at 3.0 Å), respectively, for a single H2 molecule using the revPBE-vdW functional. The negative values of adsorption energy confirm that H2 adsorption is a spontaneous process for the pristine h-BN sheet. In our previous work [65], the calculated adsorption energy (height) of a single H2 molecule over the B, N and center position is − 65.4 meV (at 3.0 Å), − 66.9 meV (at 3.0 Å) and − 67.5 meV (at 3.0 Å), respectively, for a pristine 2 × 2 × 1 monolayer h-BN substrate using the same revPBE-vdW functional. Using the kinetic Monte-Carlo simulation code [10, 65], earlier it was shown that the H2 molecules can adsorb, desorb and diffuse stochastically over the h-BN sheet [65] depending on the external temperature and pressure. The adsorption–desorption process is reversible with temperature provided the host material and gas-pressure remain constant. In the recent times, such vdW-DFT framework with revPBE-vdW functional was opted by several research groups to study the gas adsorption over different monolayer and bilayer heterostructures [66, 67].
Adsorption of H2 molecule over the defect-induced monolayer of h-BN
For the present work, four types of defect-induced systems have been considered. Boron vacancy (VB) or nitrogen vacancy (VN) is created by removing one B or N atom from the two individual pristine 5 × 5 × 1 h-BN supercells. Similarly, for a C-doped system, one carbon atom is substituted at the B (CB) or N (CN) site in the two individual 5 × 5 × 1 pristine h-BN supercells. The defect-induced structures are again geometrically optimized with revPBE-vdW functional. The average B–N bond length near the B or N vacancy positions is calculated to be 1.41 and 1.46 Å, respectively. In a doped system, the average CB or CN bond length is found to be around 1.41 and 1.50 Å, respectively. The interaction energies of a H2 molecule, \({E}_{interaction}\), over the VB, VN, CB and CN positions are calculated by following the same methodology as described in the earlier sub-Sect. "Adsorption of a single H2 molecule over a pristine h-BN system". The interaction energy curve for different defect-induced systems is given in Fig. 1c.
From Fig. 1b and c, the \({E}_{\text{adsorption}}\) for the VB and CB configuration is higher compared to the pristine, VN or CN configuration. The H2 adsorption energy is increased significantly over the defect position for the CB and VB configuration while it has been decreased for VN site and remains almost same for over the CN position. Bader charge analysis [62] is performed to understand the charge transfer from the H2 molecule to the substrates. In case of the pristine h-BN system, when the H2 molecule is adsorbed over the B and N atom, the charge transferred from the H2 molecule to the system is 0.0093 and 0.0092 e, respectively. The previously reported values of charge transfer by the H2 molecule to the B and N site of a 2 × 2 × 1 pristine h-BN monolayer are 0.0094 and 0.0093 e, respectively [65]. For adsorption over CB, CN, VB and VN site, the charge transfer value reduces to 0.0074, 0.0067, 0.0072 and 0.0042 e. Such small values of charge transfer are indicative of a weak physisorption. Also, for every case, the H2 molecule acts as a charge donor, while the substrate acts as a charge acceptor in the adsorbed state. The H2 desorption temperature (\({T}_{\text{D}}\)) can be calculated by using the Van’t Hoff equation given below [12]:
where \({E}_{D}\) has the same magnitude of \({E}_{\text{adsorption}}\), but with opposite sign. \(R\), \({k}_{B}\), \(\Delta S\) and \(P\) are, respectively, the gas constant, Boltzmann constant, change in the entropy of H2 from gaseous to liquid phase and pressure (\(P=\) 1 atmospheric pressure), respectively. The two highest desorption temperatures are obtained for the H2 adsorption over CB and VB with values 108 K and 103 K, respectively. Another important quantity, the recovery time (τ), that is the time required for a gas molecule to detach itself from the substrate is computed by using the following equation [31, 68]
with \({\nu }_{0}\) being the attempt frequency (1012 Hz at room temperature), using Boltzmann constant \({k}_{B}\) and \(T=\) 300 K, the recovery time for a H2 molecule from CB and VB is 25 and 22 ps, respectively.
Next, the impact of defect creation on the adsorption energy of the nearest neighbor and the second nearest neighbor locations is studied thoroughly by placing the H2 molecule over different positions. The defective h-BN substrate indicating the defect sites and their nearest neighbor locations is depicted in Fig. 2a to d. The adsorption height (\({d}_{0}\)) and energy (\({E}_{\text{adsorption}}\)) for different adsorption sites in the defect incorporated systems are tabulated in Table 1.
The increase in the adsorption energy is maximum for the CB substitution. Further, to understand the diffusion behavior of the adsorbed H2 molecule, one CB configuration system is chosen. The gradual change in adsorption energy is studied over the defect site and the nearest neighbor sites in the following manner. Several points are considered over the three straight lines (are shown with small black squares in Fig. 3a) connecting the defect location CB (shown in Fig. 3a) to the first nearest neighbor boron, nitrogen and second nearest neighbor nitrogen site (B1, N1 and N2 in Fig. 3a) inside the hexagonal ring.
a Schematic diagram of the diffusion path, and b diffusion barrier (using revPBE-vdW functional) experienced by a single H2 molecule from the defect site CB to various neighboring sites. The B, N and C atoms are represented by the green, gray and brown spheres, respectively. c Interaction energy curve (using revPBE-vdW functional) for the placement of a second H2 molecule above the neighboring site when a H2 molecule is already adsorbed over the CB site.
From Fig. 3b, the change in adsorption energy is smoothly increasing while moving toward the first or second nearest neighbor sites from the defect site. The H2 atom that is already adsorbed over the C position has to cross a barrier height of 13.5, 17.8 and 17.4 meV for hopping to the nearest neighbor nitrogen (N1), boron (B1) and second nearest neighbor nitrogen (N2) position, respectively.
From the above discussion, if a C-doped h-BN monolayer is suddenly exposed to a H2 flux, the first adsorption events are most likely to occur over CB position. Those first adsorbed molecules can play a pivotal role in determining the adsorption locations of the H2 molecules which are coming later. To understand this situation clearly, we place a H2 molecule above 2.8 Å over the CB site (position CB in Fig. 3a). A second H2 molecule is first placed 6 Å above four different positions individually over the h-BN sheet (positions B1, N1, N2 and C* in Fig. 3a). The vertical distance versus interaction energy curve for the second H2 molecule is calculated using revPBE-vdW functional and depicted in Fig. 3c.
When a H2 molecule is already adsorbed at the CB position, the interaction energy curve is repulsive in nature for the placement of the second H2 molecule above the nearest neighbor nitrogen (H2 at N1 in Fig. 3c) or center (H2 at C* in Fig. 3c) locations. But, for placement over the nearest neighbor boron (H2 at B1 in Fig. 3c) or the second nearest neighbor nitrogen (H2 at N2 in Fig. 3c), the interaction energy curve is again van der Waals type. The maximum adsorption energy value for the second H2 molecule is about − 58.5 and − 65.5 meV for adsorption over B1 and N2 site, respectively. It is to be noted that, in all the four cases, the two H2 molecules show huge tilting in the optimized structures as a proof of the repulsive lateral interaction going on between them. But, for H2 at B1, and N2 cases (shown in Fig. 3c), the H2 molecules can manage to orient themselves in such a way that the van der Waals interaction curve is revived.
Adsorption of a H2 molecule over a C cluster
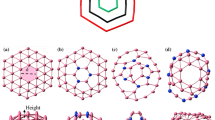
From a single substitution of C atom in a 5 × 5 × 1 supercell, we have moved on to multiple C atom substitutions. In Fig. 4a to f, a hierarchical complex formation of substitutional C defects from simple point defects is shown. In the recent work by Huang et al. [48], such C defect library over h-BN monolayer system was considered with a discussion on the stability and electronic properties of the same. It was shown that the merging of two elementary defects is energetically favorable because of a mechanism with inter-defect electron pairing [48]. For our work, the H2 molecule is placed above a particular C atom (marked using yellow highlight) within various type of C cluster and the adsorption energies are calculated using the same methodology described in the sub-Sect. "Adsorption of a single H2 molecule over a pristine h-BN system". The adsorption energies are tabulated in Table 2.
From Table 2, when a C cluster is being formed surrounding CB, the adsorption energy of a single H2 molecule is decreasing gradually, but while the cluster formation is being formed in the neighborhood of CN, the adsorption energy is gradually increasing. But, overall, the range of adsorption energy lies around 65 to 80 meV which is more than adsorption over the pristine system.
Conclusion
This present study, using revPBE-vdW functional in vdW-DFT framework, shows that the adsorption energy of single H2 molecule is significantly increased due to the presence of defects, such as C substitution at boron site (CB) and boron vacancy (VB) formation in a pristine h-BN monolayer substrate. While, the adsorption energy reduces due to the C substitution at nitrogen site (CN) and nitrogen vacancy (VN) creation. The adsorption energy is maximum with a value − 84.1 meV over CB site. The smooth decrease of the value of the adsorption energy patterns from the defect site CB to the nearest neighbor locations indicates that the first adsorption events will take place over the CB position. After one successful adsorption over the CB site, the next adsorption can take place above the nearest neighbor boron site or the second nearest neighbor nitrogen site with maximum attractive energy of about − 58.5 and − 65.5 meV, respectively. Moreover, it is also shown that the adsorption energy value of the H2 molecule above different types of C cluster lies within the range of about 65 to 80 meV which is higher than adsorption over the pristine h-BN monolayer and thus favorable for the overall storage capacity.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Schlapbach L, Züttel A (2001) Hydrogen-storage materials for mobile applications. Nature 414:353–358
Lale A, Bernard S, Demirci UB (2018) Boron nitride for hydrogen storage. ChemPlusChem 83:893–903
Deschamps J (2020) Hydrogen storage in two-dimensional and three-dimensional materials. In: Zafeiratos S (ed) 2D nanomaterials for energy applications. Elsevier, pp 227–243
Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, Grigorieva IV, Firsov AA (2004) Electric field effect in atomically thin carbon films. Science 306:666–669
Tozzini V, Pellegrini V (2013) Prospects for hydrogen storage in graphene. Phys Chem Chem Phys 15:80–89
Baraiya BA, Som NN, Mankad V, Wu G, Wang J, Jha PK (2020) Nitrogen-decorated borophene: an empowering contestant for hydrogen storage. Appl Surf Sci 527:146852
Hussain T, Chakraborty S, Ahuja R (2013) Metal-functionalized silicene for efficient hydrogen storage. ChemPhysChem 14:3463–3466
Yang S, Liu Y, Lei G, Xie Y, Peng L, Xu H, Wang Z, Gu H (2021) A DFT study on the hydrogen storage performance of MoS2 monolayers doped with group 8B transition metals. Int J Hydrogen Energy 46:24233–24246
Si H, Peng LJ, Morris JR, Pan BC (2011) Theoretical prediction of hydrogen storage on ZnO sheet. J Phys Chem C 115:9053–9058
Ghosh S, Nath P, Moshat S, Sanyal D (2023) Theoretical calculation on adsorption of molecular hydrogen in monolayer ZnO. J Appl Phys 134:155001
Chen Y-D, Yu S, Zhao W-H, Li S-F, Duan X-M (2018) A potential material for hydrogen storage: a Li decorated graphitic-CN monolayer. Phys Chem Chem Phys 20:13473–13477
Chettri B, Patra PK, Hieu NN, Rai DP (2021) Hexagonal boron nitride (h-BN) nanosheet as a potential hydrogen adsorption material: a density functional theory (DFT) study. Surf Interfaces 24:101043
Topsakal M, Aktürk E, Ciraci S (2009) First-principles study of two- and one- dimensional honeycomb structures of boron nitride. Phys Rev B 79:115442
Ogawa S, Fukushima S, Shimatani M (2023) Hexagonal boron nitride for photonic device applications: A review. Materials 16:2005
Wickramaratne D, Weston L, Van de Walle CG (2018) Monolayer to bulk properties of hexagonal boron nitride. J Phys Chem C 122:25524–25529
Ryou J, Park J, Hong S (2017) Investigations of vacancy structures related to their growth in h-BN sheet. Nanoscale Res Lett 12:445
Berseneva N, Gulans A, Krasheninnikov AV, Nieminen RM (2013) Electronic structure of boron nitride sheets doped with carbon from first-principles calculations. Phys Rev B 87:035404
Huang B, Lee H (2012) Defect and impurity properties of hexagonal boron nitride: a first-principles calculation. Phys Rev B 86:245406
Kirchhoff A, Deilmann T, Krüger P, Rohlfing M (2022) Electronic and optical properties of a hexagonal boron nitride monolayer in its pristine form and with point defects from first principles. Phys Rev B 106:045118
Islam MdS, Ushida K, Tanaka S, Makino T, Hashimoto A (2014) Analysis of vibrational properties of C-doped hexagonal boron nitride (h-BN). Comp Mater Sci 94:225–233
Yang J, Kim D, Hong J, Qjan X (2010) Magnetism in boron nitride monolayer: adatom and vacancy defect. Surf Science 604:1603–1607
Behzad S, Chegel R (2023) Optimizing thermoelectric performance of carbon-doped h-BN monolayers through carrier concentrations and magnetic field. Sci Rep 13:19623
Alem N, Erni R, Kisielowski C, Rossell MD, Gannett W, Zettl A (2009) Atomically thin hexagonal boron nitride probed by ultrahigh-resolution transmission electron microscopy. Phys Rev B 80:155425
Sutter P, Lahiri J, Albrecht P, Sutter E (2011) Chemical vapor deposition and etching of high-quality monolayer hexagonal boron nitride films. ACS Nano 5:7303–7309
Wu Q, Park J-H, Park S, Jung SJ, Suh H, Park N, Wongwiriyapan W, Lee S, Lee YH, Song YJ (2015) Single crystalline film of hexagonal boron nitride atomic monolayer by controlling nucleation seeds and domains. Sci Rep 5:16159
Tian H, He Y, Das P, Cui Z, Shi W, Khanaki A, Lake RK, Liu J (2019) Growth dynamics of millimeter-sized single-crystal hexagonal boron nitride monoleyers on secondary recrystallized Ni (100) substrates. Adv Mater Interfaces 6:1901198
Chen T-A, Chuu C-P, Tseng C-C, Wen C-K, Wong H-SP, Pan S, Li R, Chao T-A, Chueh W-C, Zhang Y, Fu Q, Yakobson BI, Chang W-H, Li L-J (2020) Wafer-scale single-crystal hexagonal boron nitride monolayers on Cu (111). Nature 579:219–223
Kimura J, Ohkubo T, Nishina Y, Urita K, Kuroda Y (2021) Adsorption enhancement of nitrogen gas by atomically heterogeneous nanospace of boron nitride. RSC Adv 11:838–846
Al-Hamdani Y-S, Ma M, Alfè D, Anatole von Lilienfeld O, Michaelides A (2015) Communication: water on hexagonal boron nitride from diffusion Monte Carlo. J Chem Phys 142:181101
Petrushenko IK, Petrushenko KB (2019) Adsorption of diatomic molecules on graphene, h-BN and their BNC heterostructures: DFT study. Diamond and Related Mater 100:107575
Kalwar BA, Fangzong W, Soomro AM, Naich MR, Saeed MH, Ahmed I (2022) Highly sensitive work function type room temperature gas sensor based on Ti doped hBN monolayer for sensing CO2, CO, H2S, HF and NO. A DFT study, RSC Adv 12:34185–34199
Rozas S, Alcalde R, Atilhan M, Aparicio S (2019) A theoretical study on the adsorption of acid gases by boron-nitride based nanomaterials. Appl Surf Sci 480:83–95
Xia S-Y, Tao L-Q, Jiang T, Sun H, Li J (2021) Rh-doped h-BN monolayer as a high sensitivity SF6 decomposed gases sensor: a DFT study. Appl Surf Sci 536:147965
Fu P, Wang J, Jia R, Bibi S, Eglitis RI, Zhang H-X (2017) Theoretical study on hydrogen storage capacity of expanded h-BN systems. Comput Mater Sci 139:335–340
Chettri B, Patra PK, Srivastava S, Laref A, Rai DP (2021) Enhanced H2 storage capacity of bilayer hexagonal boron nitride (h-BN) incorporating van der Waals interaction under an applied external electric field. ACS Omega 6:22374–22382
Rai DP, Chettri B, Patra PK, Sattar S (2021) Hydrogen storage in bilayer hexagonal boron nitride: a first-principles study. ACS Omega 6:30362–30370
Thupsuri S, Tabtimsai C, Ruangpornvisuti V, Wanno B (2021) A study of the transition metal ion doped boron nitride nanosheets as promising candidates for hydrogen and formaldehyde adsorptions. Physica E Low Dimen Syst Nanostruct 134:114859
Shelvin SA, Guo ZX (2006) Transition-metal-doping-enhanced hydrogen storage in boron nitride systems. Appl Phys Lett 89:153104
Zhou X, Chu W, Zhou Y, Sun W, Xue Y (2018) DFT simulation on H2 adsorption over Ni-decorated defective h-BN nanosheets. Appl Surf Sci 439:246–253
Venkataramanan NS, Khazaei M, Sahara R, Mizuseki H, Kawazoe Y (2009) First-principles study of hydrogen storage over Ni and Rh doped BN sheets. Chem Phys 359:173–178
Das S, Nayak SK, Sahu KK (2020) Insight into anomalous hydrogen adsorption on rare earth metal decorated on 2-dimensional hexagonal boron nitride: a density functional theory study. RSC Adv 10:12929–12940
Naqvi SAAS, Toh P-L, Wang S-M, Lim Y-C, Ang L-S, Sim L-C (2021) Computational study of hydrogen molecules adsorption on boron nitride with/without adopted by one of elements from group IV. IOP Conf Ser Earth Environ Sci 945:012001
Hao R, Shi J, Zhu L, Ji L, Sun T, Feng S (2017) A first-principle study on adsorption of atomic hydrogen on the two-dimensional hexagonal boron nitride monolayer. Superlattices Microstruct 111:696–703
Zhang H, Tong C-J, Zhang Y, Zhang Y-N, Liu L-M (2015) Porous BN for hydrogen generation and storage. J Mater Chem A 3:9632–9637
Jhi S-H, Kwon Y-K (2004) Hydroogen adsorption on boron nitride nanotubes: a path to room-temperature hydrogen storage. Phys Rev B 69:245407
Shelvin SA, Guo ZX (2007) Hydrogen sorption in defective hexagonal BN sheets and BN nanotubes. Phys Rev B 76:024104
Sagar TC, Chinthapenta V (2020) Effect of substitutional and vacancy defects on the electrical and mechanical properties of 2D-hexagonal boron nitride. J Mol Model 26:192
Huang P, Grzeszczyk M, Vaklinova K, Watnabe K, Taniguchi T, Novoselov KS, Koperski M (2022) Carbon and vacancy centers in hexagonal boron nitride. Phys Rev B 106:014107
Wei X, Wang M-S, Bando Y, Golberg D (2011) Electron-beam-induced substitutional carbon doping of boron nitride nanosheets, nanoribbons, and nanotubes. ACS Nano 5:2916–2922
Ri M, Choe K, Kim K, Gao Y, Tang Z (2018) C-doping into h-BN at low annealing temperature by alkaline earth metal borate for photoredox activity. RSC Adv 8:42109–42115
Onodera M, Isayama M, Taniguchi T, Watnabe K, Masubuchi S, Moriya R, Haga T, Fujimoto Y, Saito S, Machida T (2020) Carbon annealed HPHT-hexagonal boron nitride: exploring defect levels using 2D materials combined through van der Waals interface. Carbon 167:785–791
Loh GC, Nigam S, Mallick G, Pandey R (2014) Carbon-doped boron nitride nanomesh: stability and electronic properties of adsorbed hydrogen and oxygen. J Phys Chem C 118:23888–23896
Gao M, Adachi M, Lyalin A, Taketsugu T (2016) Long range functionalization of h-BN monolayer by carbon doping. J Phys Chem C 120:15993–16001
Mudchimo T, Namuangruk S, Kungwan N, Jungsuttiwong S (2018) Carbon-doped boron nitride nanosheet as a promising metal-free catalyst for NO reduction: DFT mechanistic study. Appl Catal A General 557:79–88
Mondinos N, Altarawneh M, Amri A, Liew WYH, Poinern GEJ, Jiang Z-T (2023) Monatomic reactions with single vacancy monolayer h-BN: DFT studies. RSC Adv 13:30346–30357
Kresse G, Hafner J (1993) Ab initio molecular dynamics for liquid metals. Phys Rev B 47:558–561
Kresse G, Hafner J (1994) Ab initio molecular-dynamics simulation of the liquid-metal-amorphous-semiconductor transition in germanium. Phys Rev B 49:14251–14269
Kresse G, Furthmüller J (1996) Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput Mater Sci 6:15–50
Kresse G, Furthmüller J (1996) Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys Rev B 54:11169–11186
Kohn W, Sham LJ (1965) Self-consistent equations including exchange and correlation effects. Phys Rev 140:A1133–A1138
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77:3865–3868
Bader RFW (1991) A quantum theory of molecular structure and its applications. Chem Rev 91:893–928
Dion M, Rydberg H, Schröder E, Langreth DC, Lundqvist BI (2004) Van der Waals density functional for general geometries. Phys Rev Lett 92:246401
Monkhorst HJ, Pack JD (1976) Special points for Brillouin-zone integrations. Phys Rev B 13:5188–5192
Ghosh S, Nath P, Moshat S, Sanyal D (2024) Adsorption and evolution of hydrogen molecules on hexagonal boron nitride monolayer: a combined DFT and kinetic Monte-Carlo simulations study. Phys Scr 99:045913
Phung VBT, Pham BL, Duy NVA, Dang MT, Tran TN, Tran Q-H, Luong TT, Dinh VA (2024) First-principles study of highly sensitive graphene/hexagonal boron nitride heterostructures for application in toxic gas-sensing devices. RSC Adv 14:4904–4916
Enujekwu FM, Ezeh CI, George MW, Xu M, Do H, Zhang Y, Zhao H, Wu T (2019) A comparative study of mechanisms of the adsorption of CO2 confined within graphene-MoS2 nanosheets: a DFT trend study. Nanoscale Adv 1:1442–1451
Hänggi P, Talkner P, Borkovec M (1990) Reaction-rate theory: fifty years after Kramers. Rev Mod Phys 62:251–341
Acknowledgments
Sulagna Ghosh and Sudipta Moshat would like to thank HBNI, DAE (Government of India) for financial support.
Funding
Open access funding provided by Department of Atomic Energy.
Author information
Authors and Affiliations
Contributions
Sulagna Ghosh was involved in data curation, data analysis, preparation of figures, writing—original draft and writing—review and editing. Palash Nath contributed to conceptualization, formalism of methodology, validation, supervision and writing—review and editing. Sudipta Moshat performed data curation, data analysis and writing—review and editing. Dirtha Sanyal participated in conceptualization, formalism of methodology, resources, validation, supervision and writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Scott Beckman.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ghosh, S., Nath, P., Moshat, S. et al. Role of carbon substitutional and vacancy in tailoring the H2 adsorption energy over a hexagonal boron nitride monolayer: an ab initio study. J Mater Sci 59, 10877–10887 (2024). https://doi.org/10.1007/s10853-024-09807-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-024-09807-x