Abstract
Three different types of carbon nanomaterials, SWCNTs, MWCNTs and GNPs were prepared, modified, characterized, and their dispersibility behavior in three different solvents was evaluated. The carbon nanotubes were synthesized by using the well-known chemical vapor deposition method and the graphene nanoplatelets by wet physicochemical treatment techniques. Their characterization was accomplished by using various advanced techniques, such as powder X-ray diffraction and Raman spectroscopy, X-ray photoelectron spectroscopy, scanning electron microscopy and N2 adsorption at 77 K. Furthermore, the carbon nanostructures were modified via plasma treatment and wet chemical surface modification in order to enhance their dispersion characteristics, for achieving more homogenous suspensions and therefore to be remained dispersed over a reasonable period of time without any sedimentation. The effect of treatment parameters and the use of different solvents were thoroughly studied mainly by optical methods, but also by using the DIN/EN classified ISO method of oil absorption and UV–Vis spectroscopy. The enhanced dispersion rate is observed in both CNTs and GNPs materials following their surface treatment, especially when using the solvent n-methyl-2-pyrrolidone. The aforementioned studied nanomaterials are perfect candidate fillers for preparing polymeric mixed matrix membranes.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carbon nanomaterials are true wonder materials and have the potential to generate distinguished novel technologies [1]. Carbon nanotubes (CNTs), fullerenes, nanodiamonds and graphene, materials with dimension of 100 nm or less and at least one-dimensional structure, are included in this list. Their unique properties, such as large surface area, good thermal properties and extraordinary mechanical performance, have attracted a lot of interest in both industry and academia. Two of the aforementioned carbon nanomaterials, the most reported and promising, are the carbon nanotubes (CNTs) and graphene. Carbon nanotube structure was observed for the first time, from Iijima, in 1991, as a new type of finite carbon structure consisting of needle-like tubes [2]. Special properties for this kind of carbon nanomaterial have been reported, such as high thermal conductivity with values between 600 and 6000 W/(m K) [3], Young’s modulus and Tensile strength of 1200 and 150 GPa, respectively, for MWCNTs [4], and electrical conductivity of 1 × 106 S/m [5].
For the newest carbon structure, discovered in 2004 [6], graphene, a wide range of impressive properties have been reported including the following: (1) high electron mobilities of over 200,000 cm2/(V s) at electron densities of ~ 2 × 1011 cm−2 [7], high thermal conductivity of ~ 5 × 103 W/(m K) [8], impermeability to gases despite being one atom thick [9], ballistic transport of electrons [10], absorption of 2% of the light passing through it [11], and being “the strongest material ever measured” [12] with a Young’s modulus of an order of magnitude of TPa [13].
Among many other applications, the development of innovative, carbon-based nanomaterials, mixed matrix membranes are included in a “hot list” of industrial research [14, 15]. Another example of this list is the contemporary research topic, where carbon nanomaterials are used for the production of nanofluids for application in refrigerators and heat exchangers, a development that has been recently reported [16].
However, the significant van der Waals attraction and the developed polar bonds among the carbon nanomaterials combined with their hydrophobic nature are some major parameters, which subserve the agglomeration and make their good dispersion a difficult and demanding challenge and task [17, 18]. Small particles, which are connected to each other by sintered necks, finally form hard agglomerates. These structures can be destroyed only by high energy milling. Soft agglomerates are accumulations of isolated particles, which are connected to each other by attractive physical interactions like van der Waals or hydrogen bridge forces. Soft agglomerates can be disrupted into smaller particles not only by high energy milling, but also by shear forces generating mechanical stress gradients [19].
Therefore, because of the spontaneous formation of carbon structure aggregates, the dispersion study and the development of new methods for avoiding this phenomenon must be the first targets before proceeding to the following step of nanocomposite material’s fabrication. Although, in the field of colloid science, the parameters for forming a good dispersion are well established [20], the development of new nanomaterials with defined architecture and special surface properties, which must be dispersed in polar or/and non-polar solvents, requires the application of special techniques for achieving the optimum dispersion.
An examined and proven method for achieving this target involves utilizing ultrasonication. But even though this treatment can break the agglomerates apart resulting in individual carbon nanoparticles, rapid re-agglomeration can occur. In many cases [21], the final product often exhibits an agglomeration level similar to that before undergoing ultrasonic treatment. To overcome this problem, one has to reduce the van der Waals interactions in order to break the agglomerates and to prevent the re-agglomeration process [22]. The most reliable route to disperse carbon nanotubes and achieve a stable dispersion is the modification of the outer surface of the used carbon nanomaterials. This modification could be materialized even by chemical functionalization and/or by plasma treatment technique which might, either, increase the affinity to dispersants or lead to repulsion effects due to likely charges of dissociated functional groups [23]. By these methods, the reduction of the van der Waals forces is permanent and therefore the dispersion becomes more stable. Moreover, the modified surface, in which new chemical functional groups are developed, plays a critical role for the further improvement of the dispersion due to the interactions between them and the solvent or/and matrix [24].
In this work, selected carbon-based nanomaterials were characterized concerning their structural and surface properties and were also investigated regarding their dispersibility behavior in three different solvent/dispersant systems. The aforementioned studied materials are perfect candidate fillers for polymeric mixed matrix membranes preparation [25].
Experimental
Carbon nanomaterials preparation and modification
The SWCNTs and the MWCNTs were produced by chemical vapor deposition of hydrocarbon gas on iron-based catalysts [26]. The GNPs were produced by a water based milling process using defined surfactants [27]. Specifically, the initial graphite oxide (GrO) precursor is constituted by multilayered graphene oxide sheets. In order to have a homogeneous dispersion of the filler into a polymer matrix, a liquid-phase exfoliation of GrO was performed obtaining few layered porous graphene oxide nanoplatelets (GNPs). Gamma-butyrolactone (GBL) was selected as the solvent, in which the exfoliation step was conducted, due to its capacity to effectively disperse graphene oxide and at the same time dissolve the candidate polymer. The GNPs preparation was followed by oxidative surface modification by acidic treatment (o-GNPs).
In addition, surface modification of both carbon nanotube types (SWCNTs and MWCNTs) and nanoscaled graphene platelets (GNPs) materials was accomplished in order to improve their dispersibility into selected organic solvents, using combinations of distinguished dispersing techniques and integration into polymer composite material. For this surface treatment, both oxygen and plasma treatment were employed. The code samples of all studied carbon nanomaterials and the optimum treatment protocols are summarized in the following table (Table 1). The chamber of the employed plasma device had a volume of 64 L, and the applied power was up to 1 kW.
Characterization techniques
The carbon nanomaterials morphology was investigated by using a field emission scanning electron microscopy (FESEM, Jeol JSM-7401F) equipped with Gentle Beam mode (operating at 2.0 kV). X-ray diffraction technique was applied, mainly for the main Bragg peaks identification. The Raman spectra were recorded using a Renishaw InVia Raman microscope with a 2D moving stage. The static Raman spectra were recorded with the WiRE 3.1 software. The elemental surface composition and chemical binding properties of the samples were analyzed by X-ray photoelectron spectroscopy (XPS) using a PHI VersaProbe III instrument. The XPS analysis was conducted for raw and treated carbon nanomaterials at NCSR “Demokritos.” The spectra were recorded utilizing monochromatic X-rays, Al kα (15 kV, 10 mA for general spectra and 15 kV, 15 mA for highly resolved measured C 1s peaks), with a medium magnification (field of view 2) lens by selecting the slot mode. For the quantification, an average value was calculated from data measured in three different sample spots. The UV–Vis absorption spectra were observed by using the Cary 100 Conc UV–visible spectrophotometer (Varian Inc). Furthermore, for the evaluation of the porous-structural nanomaterials properties liquid nitrogen (LN2) porosimetry was performed at 77 K, using Autosorb-1, Micropore version, static volumetric system (Quantachrome Instruments). The volumetric method was used to calculate the adsorbed amount of nitrogen. Prior to each measurement, the samples were outgassed overnight at 200 °C under high vacuum (10–6 Torr), achieved by a turbomolecular pump. The specific surface area was calculated from the Brunauer–Emmett–Teller (BET) method. Furthermore, for all studied samples, combination of the NLDFT (Non-Local Density Functional Theory) and the QSDFT (Quenched Solid Density Functional Theory) models regarding to the nature of the sample (e.g., geometrically and chemically disordered carbon) was applied to fit LN2 (77 K) adsorption isotherms and to calculate pore size distribution using the commercial software ASiQwin (provided by Quantachrome instruments). For these models, a kernel for nitrogen desorption on carbon was used.
Carbon filler nanomaterials dispersion procedure
Eight dried samples were introduced to solvent vials and subjected to dispersion through bath sonication employing an ultrasound water bath cleaner (Branson 3800) for 2 h [13, 28], which was the optimum sonication time treatment as derived from UV–Vis absorption spectra. In particular, 5 mg of each powder was added to 10 ml solvent in order to have 0.5 mg/ml standard concentration. The examined solvents were the following: (1) ethanol/H2O (70/30 wt%) mixture, (2) γ-butyrolactone (GBL), and (3) n-methyl-2-pyrrolidone (NMP). For comparison, the potential dispersion technique of 4 h magnetic stirring was investigated. The progression of any solution sedimentation was observed through digital pictures captured at regular time intervals.
Results and discussion
Materials characterization
Morphology of the carbon filler nanomaterials
Images from SEM electron microscope are shown in Fig. 1a. The existence of developed carbon nanotubes was evident throughout the entire length of the holder used. As depicted, MWCNTs being in abundance have the form of ribbon complexes and entangled curved filaments with no sign of any impurities and amorphous carbon and diameters varying from 13 to 25 nm. This entanglement could be justified by the inherent van der Waals interactions presented in this type of nanofiller. In addition, as depicted in Fig. 1a, top line, following the plasma treatment, the MWCNTs retain their shape, structural dimensions and characteristics.
As observed in the middle line, samples of SWCNTs with high purity consist of a mat of oriented SWCNT fibers with a diameter 20–50 nm (bundle) and a length in the micrometer-scale.
In Fig. 1a, in bottom line, the illustrated GNPs samples assemble a material exhibiting a flake structure well-organized into wrinkled sheets (graphene layers) with a smooth surface (raw sample) and varying lateral sizes.
It could be also observed that the individual platelets show a distinctly wrinkled surface, a characteristic feature of graphene oxide sheets [29] with lateral dimensions in the range of 2–4 μm. In contrast, the treated ones appear more aggregated with sizes in the range of a few micrometers (15–23 μm). This results from the oxidation process that introduces polar groups onto the basal plane, causing sheet distortion due to attractive interactions. Moreover, as can be seen in the second image of bottom line, thick aggregated structures in the form of stacks are presented with width between 70 and 80 nm. These aggregated stacks consist of multiple graphitic layers tightly bound together by non-covalent interactions such as π–π bonding [30].
In addition, Fig. 1b presents images observed in the transmission electron microscope (TEM). It is evident that the prepared MWCNTs exhibit good uniformity and fine dispersion without the presence of aggregations. The average external diameter size ranges from about 10 to about 70 nm, whereas the number of wall layers is fluctuated from 50 to 100. The SWCNTs feature parallel straight walls and closed tips, and their diameter is observed in the range of about 1.5 nm, in good agreement with the results of Raman analysis (see Sect. “3.1.3. Raman analysis”).
Furthermore, TEM images of the GNP materials provide information about their dimensions and deeper morphological characteristics. Here, it is clear that the GNPs flakes exhibit a smooth and very thin-layered structure with lateral dimensions in micrometer size range. These images also clearly confirm the high degree of exfoliation of the GNPs sheets.
XRD analysis
Three representative raw samples (MWCNTs, SWCNTs and GNPs) were characterized by X-ray diffraction (XRD) technique, and their patterns are illustrated in Fig. 2. It can be seen that the characteristic diffraction peak of MWCNTs is obvious and broad at 2θ= 26.3° attributed to the distance between the concentric graphene sheets of MWCNTs [31], while for the other two materials the peak was shifted to 26.5° corresponding to the (002) crystalline plane. Moreover, concerning GNPs, it can be observed a high and sharp intense peak confirming their crystalline structure and purity. The interplanar spacing, calculated from the Bragg’s law, is measured at 3.35 Å, similar to the graphite’s interplanar spacing [32]. The position of the (002) peak indicates graphite three-layer ABA stacking (basic structural components, BSC) [33]. Similarly, the calculated interplanar spacing between the MWCNTs sheets is 3.43 Å. The observed reduction in the (002) peak intensity in carbon nanotubes (CNTs) samples could be elucidated by the well-aligned CNTs presentation [34]. Moreover, for the SWCNTs sample, the first-order peak at 6° could be ascribed to the rope lattice existence reflection [35]. The low peaks at 43.4°, 44.55° and 53.6° were allocated to the (100), (101) and (004) crystalline planes [36], respectively. Regarding the in-plane crystallite size (La, nm), it can be calculated by the Scherrer’s formula: La = Kλ/βcosθ, where β is the line broadening at half the maximum intensity (full width at half maximum, FWHM), K = 1.84 (shape factor) and λ = 1.54056 Å (X-ray wavelength). The calculated La was 6.2, 6.4 and 9.6 nm for MWCNTs, SWCNTs and GNPs, respectively.
Finally, the Williamson–Hall method was applied for the crystallite size (L002, nm) along the (002) direction and the lattice strain (ε) estimation, as has been successfully utilized for carbon materials [37]:
with K = 0.9 in this case. The derived values of crystallite size for the three studied cases were 3.93, 19.0 and 44.7 nm for MWCNTs, SWCNTs and GNPs, respectively. In addition, for the case of GNPs, the number of graphene layers along the c-axis can be derived from the following equation:
where N is the number of the graphene layers and d is the d-spacing for (002) peak (Å). The multilayer nature of GNPs is corroborated by the existence of approximately 130 layers.
Raman analysis
Complementary to XRD analysis, the investigation of Raman spectra allows for the estimation of the relationship between ordered crystalline and disordered carbon structures in a material. Firstly, the distinctive D and G peaks of MWCNTs at about 1350 and 1583 cm−1, respectively, are clearly evident in the Raman spectra, as presented in Fig. 3a. The D band could be referred to defects in the MWCNTs samples, such as broken sp2 bonds in the sidewalls, carbonaceous impurities with sp3 bonding [38]. The ratio of peaks D and G (ID/IG ratio) is 0.89 for the raw MWCNTs, which is an indication of both the existence of a few defects in the graphite lattice and the achievement of a relatively high degree of crystallinity in the nanotubes with negligible presence of amorphous carbon.
It is also observed that the D′ band emerged as a shoulder of the G band at approximately 1628 cm−1 originating from the top of the phonon dispersion relations and it could be assigned to the intravalley double resonance process [39, 40]. The ID/ID΄ ratio could define the type of defects present in a graphene system via a simulation process developed by Eckmann et al. [41]. The calculated ratios are 5.24, 3.86 and 3.20 for the raw MWCNTs, p1-MWCNTs and p2-MWCNTs samples, respectively. These values suggest that the defects can be attributed to a combination of on-site defects arising from out-of-plane atoms bonded to carbon atoms, describing the sp3-hybridized phase, and hopping defects originating from the distortion of carbon bonds.
In Fig. 3b, the Raman spectra of SWCNTs are illustrated. They depict eight peaks related to the graphitic structures. In p-SWCNTs sample, the D band shifts to higher wavenumber compared to the untreated one (raw SWCNTs), from ~ 1345 to ~ 1350 cm−1. Likewise, for GNPs nanomaterials (Fig. 3c), the intensive peaks at approximately 1354 cm−1 and 1587 cm−1 correspond to the D and G bands, respectively. The sharp and intense peaks of G band for SWCNTs, p-SWCNTs, GNPs and p-GNPs samples justify an almost defect-free lattice order and nearly perfect graphitic structure.
At this point, it could be mentioned that the G band is, in general, an indication of bond stretching of all pairs of sp2-hybridized carbon atoms in both rings and chains or it is the primary mode in graphene and graphite. Conversely, the D band associated with structural defects, as aforementioned, is ascribed to vibrations of disordered sp3-hybridized carbon atoms or defects in the pristine graphene structure [42]. These defects may arise from finite or nanosized graphitic planes and other forms of carbon, such as heptagon-pentagon pairs, vacancies, kinks, grain boundaries, amorphous carbon species and the presence of heteroatoms [43, 44]. It is usually referred to as the disorder band or the defect band.
Furthermore, as abovementioned, the intensity ratio of the D to G bands (ID/IG) serves as a measure of the degree of disorder. A higher ratio signifies a greater presence of defects in the material’s structure, while lower ratios indicate carbon atoms sp2 bonded with few defects or high purity [45]. The spectra analysis reveals varying ratios of ID/IG intensities for the nanomaterials. It is currently acknowledged that a high relative intensity of the D band in the Raman spectra of graphene indicates a significant reduction in the dimensions of regular (graphene) sp2-domain planes. This reduction results from extensive oxidation of graphite with subsequent reduction of graphite oxide and is further influenced by additional defects introduced during the oxidation treatment. Hence, the increase of ID/IG ratio in o-GNPs, p1-MWCNTs, p2-MWCNTs and p-SWCNTs samples can be considered as a reliable indication of the successful functionalization reaction of these carbon nanomaterials provoked by various functional groups. Specifically, in the case of o-GNPs, the oxidation of GNPs could break some of the double bonds in the hexagonal structure, thereby leading to amplify the degree of disorder [46]. In addition, the acid treatment enhances the development of functional groups, such as carbonyl and ether on GNPs surface. On the other hand, the decrease of the ID/IG ratio, as the intensity of D band has been reduced in p-GNPs samples, could be attributed to the prevailing structural order of graphene sheets over the disordered regions [47].
The features of G band can be also utilized for the La (in-plane crystallite size) estimation. Indeed, for La below ~ 10 nm, Mallet-Ladeira et al. [48] suggest the use of HWHM (half-width at half maximum) of the G band, which is independent of the excitation wavelength obeying the following correlation:
For the case of the three MWCNTs samples, the calculated crystallite size fluctuates from 7 to 7.8 nm with a minor increase observed for the treated MWCNTs. This value (7 nm) approaches adequately the value derived from the XRD analysis (Sect. “XRD analysis”). For larger La (> 10 nm), the uncertainties become significant regardless of the defined domains, whether they involve edges, defect lines, or point defects [48].
Furthermore, in inset of Fig. 3b, the radial breathing mode (RBM) Raman features (first-order Raman modes) with A1g symmetry [39, 49] are presented. These features are correlated with the coherent vibration of carbon atoms in the radial direction, simulating a “breathing” motion of the tube [49]. They can be employed for the nanotube diameter (dt) distribution estimation in the sample, as these features are unique for carbon nanotubes. Regarding the diameter-dependent frequencies of various modes, a widely recognized expression is the following:
where ω0 is the frequency of 2D graphite (cm−1), β is the coefficient (cm−1 nm), and n is the exponent (−) of the diameter dependence, respectively [49]. Based on the RBM modes, the aforementioned relation concerning the RBM peak can be expressed as: ωRBM = A/dt + B with A = 234 cm−1 nm and B = 10 cm−1. The B upshift in ωRBM derives from the tube-tube interactions presented in SWCNTs bundles. Thus, the calculated diameters of SWCNTs fluctuate between 1.34 and 1.68 nm (as also indicated by TEM images) and for p-SWCNTs the values are slightly modified (1.37–1.66 nm). At this point, it could be referred that a better characterization of the diameter distribution in the SWCNT samples could be achieved with the use of various laser lines for the Raman spectra acquisition.
As observed in Fig. 3b, the experimental SWCNT G-band exhibits two features, one at ~ 1567 (G−) and the other at ~ 1594 cm−1 (G+). For our semiconducting SWCNTs case, the G− feature correlates with vibrations of carbon atoms along the circumferential direction of the SWCNT (TO-transversal optical phonon mode) and the G+ feature is associated with carbon atom vibrations along the nanotube axis (LO-longitudinal optical phonon mode) [49]. The Lorentzian line shape justifies the semiconducting nature of SWCNTs samples. The G+ peak is independent of nanotubes diameter, and on the contrary, the G− obeys the aforementioned Eq. 4 with ω0 = 1591 cm−1, β = − 47.7 cm−1 nm and n = 2 [50] (values for semiconducting SWCNTs) resulting in the diameter calculation (approximately 1.4 nm for both SWNTs) lying in the range of the RBM modes estimation. Regarding the ratio of intensities IG−/IG+, it fluctuates between 0.32 and 0.36 for both SWCNTs samples in accordance with studied SWCNTs in the literature [50].
In addition, one set of overtone features is observed at about 1750 cm−1, as depicted in the second inset of Fig. 3b. This feature assigned to M band [49], which consists of two components M− (1736 cm−1) and M+ (1758 cm−1). These features are ascribed to overtones of the out-of-plane tangential optical (oTO) phonon, and their downshift occurrence relative to 2D graphite is owing to the diameter-dependent curvature effects [49]. The carbon nanotubes’ curvature also explains the break of peak symmetry.
The origin of the G* band at ~ 2450 cm−1 in all samples (with different intensities) is still under investigation. Previous studies assigned to the G* band to a double resonance phenomenon, in particular, to an overtone mode of LO (longitudinal optical) phonon near the K-points [51, 52] or a combination of both optical and acoustic phonon modes [52,53,54], where the latter is defined as incoherent oscillations of atoms from their equilibrium positions. Then, in the Raman spectra for SWCNTs samples (Fig. 3b), the 2G band, as overtone of G model located at 3180 cm−1, is appeared due to two-phonon, second-order double resonance process [49]. Generally, the observation of overtones in condensed matter systems is rare, as dispersion effects make these features too weak and broad [49]. Notwithstanding, the presence of van Hove singularities in SWCNTs and the double resonance process eventually led to the aforementioned band observation.
Two additional defect-induced modes of lower intensity are also observed (Fig. 3a). The D′ band appearing as a shoulder to the G mode at ~ 1620 cm−1 arises from a double resonance process (inelastic phonon scattering near a crystal defect) similar to the D band (the presence of structural defects inside graphene layer). The D΄ band is not visible in the Raman spectra of all samples, but is invariably present in G band of Raman spectra of MWCNTs. The relatively broad D + G combination mode at ~ 2940 cm−1 is presented for MWCNTs group, p-SWCNTs and o-GNPs.
Finally, the G′ or 2D band, overtone of the D band [55], is consistently observed at ∼2706 cm−1 in all materials. It is due to a phonon–phonon inelastic scattering process (second-order Raman scattering) [55, 56]. Its sensitivity to the stacking order of graphitic walls and the crystallinity of graphitic planes is noteworthy and its symmetry provides insights into the number of layers present. A single graphene layer produces a symmetric peak with I2D/IG ratio equal to 2 [56] with the symmetry diminishing as the number of layers increases. Τhe calculated I2D/IG intensity ratios are below one (GNPs samples) indicating the multilayer structure of GNPs samples. The lower values of the treated GNPs ratio (0.12 and 0.44 for o-GNPs and p-GNPs, respectively) compared to the raw sample value (0.52) suggest the increase in the stacked graphene layers number. For the o-GNPs analyzed in this work, the 2D band is slightly decentered and broadened with decrease in its intensity suggesting an exfoliated flake structure and high degree of functionalization. Similarly, the slight reduction in intensity of I2D from 0.44 (raw GNPs) to 0.41 (p-GNPs) establishes the functionalization confirmation. For the case of MWCNTs, the reduced value of the I2D/IG ratio in raw MWCNTs (0.83) is possibly ascribed to sp3 bonding defects, kinks and twists in the structure of the carbon nanotubes [45]. This is in contrast to the plasma-treated ones, where higher values of 0.86 and 0.96, respectively, imply the impact of a “healing-effect” on the structural integrity of the carbon nanotubes. Eventually, in Fig. 3b, the 2D peak is much more intense than the D peak claiming the good quality of the SWCNTs samples, as aforementioned the scattering events are induced only from phonons formation [57].
In order to have better and integrated insight about the type of SWCNTs, different excitation laser lines should be employed, as the electronic density of states of SWCNTs exhibit van Hove singularities [58] (dependence of energy positions on nanotube diameter and semiconductor or metallic nature).
Summarizing it could be referred that all the above-mentioned calculations are indicative and more advanced and robust techniques, such as electron diffraction (ED), high-resolution transmission electron microscopy (HRTEM) or scanning tunneling microscopy (STM), must be employed in order to shed light better on structural characteristics of SWCNTs and MWCNTs.
XPS analysis
In Fig. 4a–c, the XPS surveys curves of the three studied types of nanocarbon materials are illustrated. It is observed that carbon and oxygen are primarily present on the surface of all samples assigned to the binding energy of approximately 285 and 532 eV, respectively. The oxygen atoms in the nanocarbon materials can be attributed to their formation during the preparation process, physically adsorbed oxygen or oxygenated functional groups bonded to the defective sites [59]. Moreover, the peaks presented as O KLL (Fig. 4a-c) at a range of 974–975 eV depict the energy of the electrons ejected from the atoms due to the filling of the O 1s state (K shell) by an electron from the L shell coupled with the ejection of an electron from an L shell.
As observed in Fig. 4d, the deconvolution of the C 1s core level spectrum of raw MWCNTs reveals the presence of four peaks relevant with sp2 C=C, sp3 C–C and epoxy or hydroxyl C–O bonds located at 284.8, 285.4 and 287.0 eV, respectively [60]. In addition, the π–π* shake-up satellite peak located at ~ 291 eV is presented [61]. Concurrently, the oxygen 1s (core level spectrum) of p2-MWCNTs sample (Fig. 4e) can be deconvoluted into two distinct peaks assigned to the C–O bond (532.0 eV) and C=O bond (533.7 eV).
In addition, the treatment conditions as well as the results from the XPS spectra analysis in order to identify the chemical composition of samples’ surface and any potential changes in their surface characteristics are listed in Table 2. A clear increase in the O content in all treated samples is observed, as indicated by the ratio between the signals of C1s and O1s.
Initially, concerning the first employed plasma device (Diener Plasma/p1-MWCNTs), it is concluded that both operational parameters, that is applied power and time duration of plasma treatment process mainly influences the oxygen concentration in the final output with higher and more prominent impact to possess the elongation of treatment duration (data not shown). Consequently, the manipulation of operational parameters during plasma treatment leads to the tuning of the oxygen group distribution on the surface of carbon nanotubes.
Then, the surface’s oxygen ratio O/C of the plasma-treated MWCNTs was significantly enhanced (from 0.44 for raw to 11.54 and 4.71 for p1-MWCNTs and p2-MWCNTs, respectively). As a result, the plasma-treated MWCNTs showcased a higher presence of oxygen functional groups on their surfaces compared to the untreated MWCNTs.
As outlined in Table 2, the sp2/sp3 ratio was additionally evaluated. In particular, by determining the percentages of sp2 and sp3, excluding the remaining carbon related species, these ratios were derived from the following relationships:
It is worth noting that in theoretically ideal MWCNTs, carbon atoms arranged in hexagonal shapes and covalently bonded to three other carbon atoms are exclusively composed of sp2-orbitals. However, in reality, defects in the carbon nanotube lattice manifest in the form of the sp3 hybridization. Indeed, the higher the sp2/sp3 ratio, the fewer defects exist in the molecular grid of the MWCNTs. Thus, as indicated in Table 2, the increased sp2/sp3 ratio following plasma treatment of MWCNTs compared to the raw ones signifies a “healing-effect” on the carbon nanotubes, underscoring the significance of the process. This consistent trend was also corroborated by the Raman analysis in Sect. “Raman analysis.”
As outlined in Sect. “Carbon nanomaterials preparation and modification,” the second plasma device from SurfaceTreat employed an automatic system with a rotating jar for mixing implementation. Despite the lower oxygen atomic concentration in the p2-MWCNTs sample compared to the p1-MWCNTs sample, the resulting sp2/sp3 ratio is about 6%, surpassing that observed in the p1-MWCNTs sample.
Finally, wet-chemically oxidized GNPs (subjected to KMnO4 treatment) were also investigated and analyzed using XPS technique, as illustrated in Table 2. The results revealed a remarkably high oxygen content of 27.41%, significantly exceeding that observed in the plasma-treated ones (6.1%).
Overall, the enhancement in the O/C ratio following either plasma or oxidized treatment indicates the substantial impact of oxygen during the treatment process.
Alternatively, the oxygen content on the surface of the modified carbon nanomaterials was determined using the acid–base titration technique, yielding results consistent with the observations from XPS.
Regarding the percentages of carbon–oxygen single bonds (C–O) and carbon bonded to oxygen through double bonds (C=O) species obtained through the deconvolution of the O-1s orbitals and presented in Table 2, alkoxy groups were found to prevail over carbonyl groups in the modified samples in comparison with the raw ones.
LN2 porosimetry
The pore structural and textural properties of all carbon nanomaterials were investigated by liquid N2 (LN2) porosimetry at 77 K (Fig. 5). The samples of MWCNTs group exhibited type IV N2 adsorption isotherms, with a H1-type hysteresis loop implying a material with well-defined cylindrical pores [62]. They also presented a sharp capillary condensation step at high relative pressure of P/P0 > 0.97, which, according to the Kelvin equation, is characteristic of materials containing large mesopores and macropores. Analyzing the three derived isotherms for the MWCNTs group, the differences between the plasma-treated MWCNTs and the untreated one were minor. The modified MWCNTs samples, resulting from different plasma treatments, exhibited a similar pore structure, albeit with a less pronounced hysteresis evident in the isotherms. Their BET (Brunauer–Emmett–Teller) surface areas (SBET) were ranged from 193 to 215 m2/g, and their measured total pore volumes (TPV) fluctuated between 0.61 and 0.63 ml/g (Table 3). Straightaway it could be referred to Peigney et al. [63] who explored the theoretical external specific surface area (s.s.a.) as a function of the characteristics of CNTs (diameter, number of walls and nanotubes in a bundle). Their findings, illustrated in diagrams and tables, suggest an average of 14 nm in external diameter for carbon nanotubes with a wall count ranging between 5 and 10. In order to obtain a more precise estimation of the theoretical s.s.a., it becomes imperative to define the distributions of MWCNT diameters and the numbers of walls with greater accuracy.
In addition, the LN2 adsorption/desorption sigmoid-shaped isotherms of SWCNTs group, depicted in Fig. 5b, could be classified as type I, which were characterized by a very steep initial uptake at low P/P0 (< 0.1) indicating a very strong contribution of micropores followed by a gradually increase in the adsorption uptake due to an extent to the mesoporosity existence. The observed hysteresis loop toward the end (at P/P0 > 0.9) is related to the capillary condensation of nitrogen in mesopores. In terms of their SBET and TPV, these materials exhibited the highest values among all the carbon nanomaterials, reaching up to 1310 m2/g and 1.5 ml/g, respectively. These values are remarkably comparable to the theoretical estimated surface area of 1315 m2/g for discrete SWCNTs [63].
Moreover, after the treatment of the GNPs with the oxidizing agent, the N2 adsorption/desorption isotherm of the o-GNPs exhibited a typical type IV isotherm, featuring a H4-type hysteresis loop according to IUPAC classification, which is characteristic of pore sizes located at the boundary between micropores and mesopores [64]. The extended hysteresis loop starting from high relative pressure of approximately P/P0 = 0.95 indicates a wide pore size distribution, whereas the steep drop of the desorption branch at about 0.5 (P/P0) refers to the higher population of a specific size of pores (about 3.4 nm), as depicted in Fig. 6c. Therefore, based on the nitrogen adsorption data, the o-GNPs material is characterized by a micro- to mesoporous structure. Similar behavior is noted for the other two GNP samples, which display narrower hysteresis loops. This type of hysteresis loop (H4) is frequently identified in micro/mesoporous carbons according to IUPAC classification [62].
As depicted in Fig. 6a, the MWCNTs group exhibited a broad pore size distribution (PSD) characterized by peaks in both the micropore (predominantly for the raw sample, somewhat suppressed in the plasma-treated samples, yet with the retention of micropore volume) and mesopore regions (total pores range from 0.6 up to 26 nm). Particularly, the incomplete peak below 0.6 nm in raw MWCNTs might be attributed to a small contribution from small mesopores originating from the inner channels of the carbon nanotubes. On the contrary, SWCNTs materials displayed a narrower bimodal PSD curve (Fig. 6b) with a distinct shift toward the supermicropore region (0.7–2 nm). The majority of pores exhibited sizes ≤ 2 nm, confirming the prevalence of microporosity. Finally, as presented in Fig. 6c, the raw GNPs present a bimodal PSD curve featuring one sharp bell (incomplete) in the ultramicropore area (0.6 nm) and a second broader peak in the mesoporous area extending up to approximately 17 nm. Equivalently, the p-GNPs sample displays behavior akin to that of raw GNPs but with narrower range up to 10 nm. On the other hand, the o-GNPs sample demonstrates a different bimodal PSD curve with distinct and sharp peaks located at 1.4 and 3.4 nm, respectively (comprising the majority of pores), justifying the micro/mesoporous nature of this material.
Table 3 summarizes the textural properties and pore structural characteristics of all studied samples derived by the liquid nitrogen (LN2) adsorption technique.
Study of carbon nanomaterials dispersibility
Oil absorption method
A facile method was developed to assess the surface and dispersing properties of the examined nanomaterials. The oil absorption technique, in accordance with DIN EN ISO 787-5 Standard, utilized linseed oil based on ISO 150, commonly used in the printing ink industry. Given that it involves a titration method with challenges in endpoint detection, the use of a Speedmixer (Fig. 7a) is recommended for easier and enhanced endpoint detection. In this method, the initial carbon powder was weighted into a plastic cup, a small quantity of linseed oil was added, the cup was sealed and the carbon–oil mixture is then homogenized using the Speedmixer.
This process was repeated, until the carbon/oil mixture transitioned from a crumbly, particulate state to a cohesive clump in the cup (Fig. 7b). The calculation of the oil absorption amount in ml/g was derived from the carbon powder weighing and the added linseed oil consumption.
Table 4 summarizes the results of the oil absorption for all the studied samples. Overall, minimal differences are observed in the values. The plasma-treated MWCNTs (p1-MWCNTs and p2-MWCNTs) with high O2 content display lower oil absorption, suggesting that their dispersion ability should be facilitated compared to the pristine sample.
In addition, the SWCNTs present the lowest oil absorption amount in comparison with the MWCNTs, yet their dispersing process is characterized by more complicated features, primarily owing to the formation of very large agglomerates. Conversely, for o-GNPs, it is evident that the oxidation treatment results in significantly reduced oil absorption, facilitating easier dispersion when compared to untreated GNPs.
ζ-potential and dispersibility evaluation
Immediately following their preparation, carbon nanomaterials were dispersed in three specified solvents: ethanol/water (70/30 wt) mixture (1), gamma-butyrolactone, GBL (2), and n-methyl-2-pyrolidone, NMP, (3). In addition, the eight studied samples were assigned letters A to H. Moreover, recognizing that zeta potential serves as an indicator of the colloidal particles’ dispersion properties, corresponding measurements were performed to assess and quantify the stability of the nanomaterials in these different solvents.
As described in Sect. “Carbon filler nanomaterials dispersion procedure,” two approaches of carbon nanomaterials’ dispersion were followed aiming to evaluate and shed light to their dispersibility efficiency. As such, representative digital images were captured at regular time intervals in order to facilitate a comparison between the stirring and bath sonication techniques.
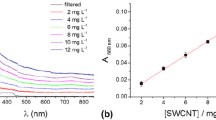
As indicated by the UV–Vis absorption spectra, the optimal sonication treatment duration was found to be 120 min (Fig. 8). In particular, Fig. 8 depicts the UV–Vis absorption spectra of the selected dispersed studied nanomaterials at 30, 60 and 120 min. During sonication, the introduced mechanical energy successfully surpasses the van der Waals interactions in the carbon nanomaterials bundles, resulting in the disruption of agglomerates and achieving a well-dispersed suspension. Here, it must be noted that there is no further difference in the UV–Vis spectra after treatment exceeding 120 min. Indeed, as shown in Fig. 8, the area beneath the corresponding spectra at 120 min is larger than those at 60 and 30 min [65]. This indicates that the most optimal suspensions are obtained through sonication treatments lasting 120 min.
Depending on the carbon nanomaterial type (MWCNTs, SWCNTs, GNPs, plasma treated, oxidized, etc.), the suspension can be tough and robust. Sonication, in contrast to mechanical stirring, aids in re-suspending the particles by mainly disrupting weak interconnection forces among nanoparticles [22, 26, 66]. Furthermore, the local energy input during stirring is considerably lower compared to the ultrasonically induced jet streams. The differences between stirring and sonication treatment are readily observable. Indeed, as shown in Fig. 9 for the samples A1, B1 and C1, the dispersions exhibit a notably poor condition immediately after the stirring process. This is absolutely discernible, when after 30 min, a complete separation between the carbon material and the solvent is observed. At the same time, the precipitation process has been also initiated for samples D1 and E1, being fully materialized at a high extent after a time period of 120 min, as clearly noticed. Fifteen hours after the stirring switched-off, only sample G1 withstand the precipitation process; but again a remarkable quantity of the suspended o-GNPs has been also precipitated. In contrast, sonication treatment imparts distinctly different characteristics to the nanomaterials’ dispersibility. The dispersed solutions of treated nanomaterials (except E1/plasma-treated SWCNTs) remain stable even 15 h after sonication, and concurrently, the treated nanomaterials exhibit a significantly improved state compared to their raw ones, showcasing enhanced stability and a more favorable appearance.
For the case of stirring, the dispersion was sufficiently enhanced by substituting the solvent with gamma-butyrolactone, GBL, an aprotic solvent (Fig. 10).
In this case, samples D2, E2 and G2 exhibit acceptable stability not only after 120 min, but also after 15 h. On the other hand, sonication enables a higher number of nanomaterials to achieve well-dispersed states in GBL. Specifically, samples B2, C2, E2 and G2 depict a highly stable and non-precipitated appearance.
Finally, when the solvent was replaced by NMP (also an aprotic solvent), there was a noticeable improvement in the dispersions of all studied nanomaterials (Fig. 11). Bath sonication treatment yielded even better results for almost all the studied nanomaterials. As outlined in Table 2, the resulting C/O ratios for samples B, C, G and H exceeded those of their precursors. In addition, their ζ-potential values categorize them as systems with “Threshold of agglomeration,” “Moderate stability” and “Fairly good stability” according to the classification in Table 6.
A prolonged assessment of nanomaterials’ dispersibility is depicted in Fig. 12. Samples C, E and, to some extent, G demonstrated enduring stability over a 30-day period in GBL solvent. Additionally, these samples, along with sample B, showcased exceptional long-term stability in NMP solvent.
Overall, it could be presumed that the developed functional groups on the modified carbon nanomaterials contribute beneficially toward their dispersibility efficiency in polar aprotic solvents such as GBL and NMP. Based on these primitive observations and results, oxidized GNPs and plasma-treated MWCNTs, in particular, emerge as competent and promising nanofillers for dual-layer composite and/or mixed matrix membranes fabrication, as already demonstrated in our recently published works [25, 67].
Furthermore, it could be referred that a property crucial for defining the stability of a suspension is the zeta potential, when the dispersing mechanism relies on repulsive local charges [68,69,70]. Following, Table 5 elucidates this relationship based on the aforementioned work [69].
In order to investigate the dispersion characteristics of the eight prepared solutions, labeled A-H, zeta potential measurements were conducted using a zeta potential analyzer, a dynamic light scattering (DLS) instrument. The results are illustrated in Table 6. The substantial variations in zeta potential values for each studied sample are obvious, and the corresponding images in Fig. 9 are distinctly correlated with these ζ-potential values.
In Table 6, the ζ-potential properties are mentioned specifically for nanomaterial suspensions in an ethanol/water (70/30 wt%) mixture. A technical restriction stemming from the solvent sensitivity of the DLS (dynamic light scattering) cuvette-cell prevented obtaining any ζ-potential information for nanomaterial suspensions in GBL and NMP solvents. The samples described in Table 6 correspond to those presented in Fig. 9.
In specific, a high value (absolute value) of zeta potential correlates with a stable dispersion, categorizing the dispersed carbon nanomaterials as “well dispersed.” On the other hand, a low zeta potential illustrates an attractive force, notably stronger than the repulsive force, leading to the tendency of dispersion to flocculate. The functional groups introduced during the treatment process facilitate the carbon nanomaterials to be dispersed. For instance, the polar solvent, the water/ethanol mixture, achieves the dispersion of GNPs sheets through solvent exchange from water [71]. In general, it is demonstrated that at a low potential, attractive forces may surpass repulsive ones, resulting in coagulation and flocculation of the dispersion. Conversely, a high zeta potential (in absolute value) electrically stabilizes the colloid system [72].
Conclusions
The importance of stable dispersions for the well-dispersed mixed matrix membrane preparation cannot be overstated. In this work, three raw carbon nanomaterials, MWCNTs, SWCNTs and GNPs, as well as five modified derivatives from them were characterized concerning selected physical chemistry properties and their stability in three different solvents was also studied and discussed. It is clear that both O2 plasma treatment and the oxidation with KMnO4 introducing oxygen functional groups on the nanomaterials’ surface enhanced their dispersibility. Employing a straightforward sonication process, the suspensions of the eight nanomaterials under investigation remained stable in GBL and NMP solvents for a time of 30 min. However, in the case of Ethanol/H2O solvent, only the GNPs suspensions were stable within this time frame.
All the examined nanomaterials exhibit porous characteristics, with a specific surface area (s.s.a.) ranging from about 40–1310 m2/g and porosity spanning 11–73%. Notably, the SWCNTs, despite presenting the highest s.s.a. and pores size of ~ 7 Å, demonstrated the poorest dispersibility due to the inability of the solvent to penetrate their pore network. Overall, the studied nanomaterials are good and promising candidates as membrane filler materials. The assessment of their dispersibility study in various solvents, with emphasis in new, green and environmentally friendly solvents, provides valuable insights for the membrane research community. Therefore, there is merit in continuing and expanding this research, especially for modern nanomaterials and green solvents, such as GBL. In this context, an ongoing molecular dynamics (MD) simulation extensively explores nanomaterials’ dispersion in various solvents, aiming to elucidate to interactions mechanisms between fillers and solvents, thus contributing to advancements in their dispersibility.
Data and code availability
All the presented data are original and available under request.
References
Rashid SA, Othman RNIR, Hussein MZ (2018) Synthesis, technology and applications of carbon nanomaterials, micro and nano technologies. Elsevier, Netherlands
Iijima S (1991) Helical microtubules of graphitic carbon. Nature 354:56–58
Yang DJ, Zhang Q, Chen G, Yoon SF, Ahn J, Wang SG, Zhou Q, Wang Q, Li JQ (2002) Thermal conductivity of multiwalled carbon nanotubes. Phys Rev B 66:165440
Seetharamappa J, Yellappa S, D’Souza F (2006) Carbon nanotubes. Electrochem Soc Interface 15(2):23–26
Shoukat R, Khan MI (2021) Carbon nanotubes: a review on properties, synthesis methods and applications in micro and nanotechnology. Microsyst Technol 27:4183–4192
Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, Grigorieva IV, Firsov AA (2004) Electric field effect in atomically thin carbon films. Science 306:666–669
Bolotin KI, Sikes KJ, Jiang Z, Klima M, Fudenberg G, Hone J, Kim P, Stormer HL (2008) Ultrahigh electron mobility in suspended graphene. Solid State Commun 146:351–355
Balandin AA, Ghosh S, Bao W, Calizo I, Teweldebrhan D, Miao F, Lau CN (2008) Superior thermal conductivity of single-layer graphene. Nano Lett 8:902–907
Bunch JS, Verbridge SS, Alden JS, van der Zande AM, Parpia JM, Craighead HG, McEuen PL (2008) Impermeable atomic membranes from graphene sheets. Nano Lett 8:2458–2462
Du X, Skachko I, Barker A, Andrei EY (2008) Approaching ballistic transport in suspended graphene. Nat Nanotechnol 3:491–495
Nair RR, Blake P, Grigorenko AN, Novoselov KS, Booth TJ, Stauber T, Peres NMR, Geim AK (2008) Fine structure constant defines visual transparency of graphene. Science 320:1308
Lee C, Wei X, Kysar JW, Hone J (2008) Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 321:385–388
Johnson DW, Dobson BP, Coleman KS (2015) A manufacturing perspective on graphene dispersions. Curr Opin Colloid Interface Sci 20:367–382
Guo X, Li C, Li C, Wei T, Tong L, Shao H, Zhou Q, Wang L, Liao Y (2019) G-CNTs/PVDF mixed matrix membranes with improved antifouling properties and filtration performance. Front Environ Sci Eng 13:81
Ho KC, Teow YH, Ang WL, Mohammad AW (2017) Novel GO/OMWCNTs mixed-matrix membrane with enhanced antifouling property for palm oil mill effluent treatment. Sep Purif Technol 177:337–349
Okonkwo EC, Wole-Osho I, Almanassra IW, Abdullatif YM, Al-Ansari T (2021) An updated review of nanofluids in various heat transfer devices. J Therm Anal Calorim 145:2817–2872
Sabri FNAM, Zakaria MR, Akil HM (2020) Dispersion and stability of multiwalled carbon nanotubes (MWCNTs) in different solvents. AIP Conf Proc 2267:020043
Zhang Q, Wang YC, Bailey CG, Istrate OM, Li Z, Kinloch IA, Budd PM (2019) Quantification of gas permeability of epoxy resin composites with graphene nanoplatelets. Compos Sci Technol 184:107875
Liu Y, Gao X, Qian W, Wang Y, Wei F (2011) Architectural and mechanical performances of carbon nanotube agglomerates characterized by compaction response. Powder Technol 211:226–231
Everett DH (1988) Basic principles of colloid science. The Royal Society of Chemistry, London
Yue L, Pircheraghi G, Monemian SA, Manas-Zloczower I (2014) Epoxy composites with carbon nanotubes and graphene nanoplatelets–dispersion and synergy effects. Carbon 78:268–278
Favvas EP, Stefanopoulos KL, Stefopoulos AA, Nitodas SF, Mitropoulos ACh, Lairez D (2016) Phenol functionalized MWCNTs: a dispersion study into polar solvents by small angle neutron scattering. Colloids Surf, A Physicochem Eng Asp 496:94–99
Dongil AB, Bachiller-Baeza B, Guerrero-Ruiz A, Rodríguez-Ramos I, Martínez-Alonso A, Tascón JMD (2011) Surface chemical modifications induced on high surface area graphite and carbon nanofibers using different oxidation and functionalization treatments. J Coll Interf Sci 355:179–189
Spitalsky Z, Tasis D, Papagelis K, Galiotis C (2010) Carbon nanotube–polymer composites: chemistry, processing, mechanical and electrical properties. Progr Polym Sci 35:357–401
Theodorakopoulos GV, Karousos DS, Mansouris KG, Sapalidis AA, Kouvelos EP, Favvas EP (2022) Graphene nanoplatelets based polyimide/pebax dual-layer mixed matrix hollow fiber membranes for CO2/CH4 and He/N2 separations. Int J Greenh Gas Control 114:103588
Favvas EP, Nitodas SF, Stefopoulos A, Stefanopoulos KL, Papageorgiou SK, Mitropoulos ACh (2014) High purity multi-walled carbon nanotubes: preparation, characterization and performance as filler materials in co-polyimide hollow fiber membranes. Sep Purif Technol 122:262–269
Ba TL, Mahian O, Wongwises S, Szilágyi IM (2020) Review on the recent progress in the preparation and stability of graphene-based nanofluids. J Therm Anal Calorim 142:1145–1172
Paredes JI, Villar-Rodil S, Martínez-Alonso A, Tascón JMD (2008) Graphene oxide dispersions in organic solvents. Langmuir 24:10560–10564
Bandeira P, Monteiro J, Baptista AM, Magalhães FD (2016) Influence of oxidized graphene nanoplatelets and [DMIM][NTf2] ionic liquid on the tribological performance of an epoxy-PTFE coating. Tribol Intern 97:478–489
McLaren RL, Laycock CJ, Brousseau E, Owen GR (2021) Examining slit pore widths within plasma-exfoliated graphitic material utilising Barrett–Joyner–Halenda analysis. New J Chem 45:12071–12080
da Silva VA, Rezende MC (2018) Effect of the morphology and structure on the microwave absorbing properties of multiwalled carbon nanotube filled epoxy resin nanocomposites. Mater Res 21:e20170977
Howe JY, Rawn CJ, Jones LE, Ow H (2003) Improved crystallographic data for graphite. Powder Diffr 18:150–154
Puech P, Dabrowska A, Ratel-Ramond N, Vignoles GL, Monthioux M (2019) New insight on carbonisation and graphitisation mechanisms as obtained from a bottom-up analytical approach of X-ray diffraction patterns. Carbon 147:602–611
Cao A, Xu C, Liang J, Wu D, Wei B (2001) X-ray diffraction characterization on the alignment degree of carbon nanotubes. Chem Phys Lett 344:13–17
Rinzler AG, Liu J, Dai H, Nikolaev P, Huffman CB, Rodríguez-Macías FJ, Boul PJ, Lu AH, Heymann D, Colbert DT, Lee RS, Fischer JE, Rao AM, Eklund PC, Smalley RE (1998) Large-scale purification of single-wall carbon nanotubes: process, product, and characterization. Appl Phys A Mater Sci Process 67:29–37
Wang Q, Wang Y, Meng Q, Wang T, Guo W, Wu G, You L (2017) Preparation of high antistatic HDPE/polyaniline encapsulated graphene nanoplatelet composites by solution blending. RSC Adv 7:2796–2803
Mauro M, Cipolletti V, Galimberti M, Longo P, Guerra G (2012) Chemically reduced graphite oxide with Improved shape anisotropy. J Phys Chem C 116:24809–24813
DiLeo RA, Landi BJ, Raffaelle RP (2007) Purity assessment of multiwalled carbon nanotubes by Raman spectroscopy. J Appl Phys 101:1–5
Saito R, Grüneis A, Samsonidze GG, Brar VW, Dresselhaus G, Dresselhaus MS, Jorio A, Cançado LG, Fantini C, Pimenta MA, Filho AGS (2003) Double resonance Raman spectroscopy of single-wall carbon nanotubes. New J Phys 5(1):1–157
Hou X, Sheng L, Yu L, An K, Ando Y, Zhao X (2012) Surface-enhanced Raman spectra of individual multiwalled carbon nanotubes with small innermost diameters. J Raman Spectrosc 43:1381–1384
Eckmann A, Felten A, Mishchenko A, Britnell L, Krupke R, Novoselov KS, Casiraghi C (2012) Probing the nature of defects in graphene by Raman spectroscopy. Nano Lett 12:3925–3930
Ferrari AC (2007) Raman spectroscopy of graphene and graphite: disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun 143:47–57
Prolongo SG, Jiménez-Suárez A, Moriche R, Ureña A (2014) Graphene nanoplatelets thickness and lateral size influence on the morphology and behavior of epoxy composites. Eur Polymer J 53:292–301
Yang S-Y, Lin W-N, Huang Y-L, Tien H-W, Wang J-Y, Ma C-CM, Li S-M, Wang Y-S (2011) Synergetic effects of graphene platelets and carbon nanotubes on the mechanical and thermal properties of epoxy composites. Carbon 49:793–803
Zakaria MR, Kudus MHA, Akil HMd, Thirmizir MZM (2017) Comparative study of graphene nanoparticle and multiwall carbon nanotube filled epoxy nanocomposites based on mechanical, thermal and dielectric properties. Compos B 119:57–66
Khan NI, Halder S, Talukdar N, Das S, Goyat MS (2021) Surface oxidized/silanized graphite nanoplatelets for reinforcing an epoxy matrix. Mater Chem Phys 258:123851
Kumar N, Srivastava VC (2018) Simple synthesis of large graphene oxide sheets via electrochemical method coupled with oxidation process. ACS Omega 3:10233–10242
Mallet-Ladeira P, Puech P, Toulouse C, Cazayous M, Ratel-Ramond N, Weisbecker P, Vignoles GL, Monthioux M (2014) A Raman study to obtain crystallite size of carbon materials: a better alternative to the Tuinstra-Koenig law. Carbon 80:629–639
Dresselhaus MS, Dresselhaus G, Saito R, Jorio A (2005) Raman spectroscopy of carbon nanotubes. Phys Rep 409:47–99
Jorio A, Souza Filho AG, Dresselhaus G, Dresselhaus MS, Swan AK, Ünlü MS, Goldberg BB, Pimenta MA, Hafner JH, Lieber CM, Saito R (2002) G-band resonant Raman study of 62 isolated single-wall carbon nanotubes. Phys Review, B 65:155412
Shimada T, Sugai T, Fantini C, Souza M, Cançado LG, Jorio A, Pimenta MA, Saito R, Grüneis A, Dresselhaus G, Dresselhaus MS, Ohno Y, Mizutani T, Shinohara H (2005) Origin of the 2450 cm−1 Raman bands in HOPG, single-wall and double-wall carbon nanotubes. Carbon 43:1049–1054
Saito R, Hofmann M, Dresselhaus G, Jorio A, Dresselhaus MS (2011) Raman spectroscopy of graphene and carbon nanotubes. Adv Phys 60:413–550
Mafra DL, Samsonidze G, Malard LM, Elias DC, Brant JC, Plentz F, Alves ES, Pimenta MA (2007) Determination of LA and TO phonon dispersion relations of graphene near the dirac point by double resonance Raman scattering. Phys Rev B 76:233407
Saito R, Sato K, Araujo PT, Mafra DL, Dresselhaus MS (2013) Gate modulated Raman spectroscopy of graphene and carbon nanotubes. Solid State Commun 175–176:18–34
Lehman JH, Terrones M, Mansfield E, Hurst KE, Meunier V (2011) Evaluating the characteristics of multiwall carbon nanotubes. Carbon 49:2581–2602
Bataklie T, Petrova-Doycheva I, Angelov V, Georgiev V, Ivanov E, Kotsilkova R, Casa M, Cirillo C, Adami R, Sarno M, Ciambelli P (2019) Effects of graphene nanoplatelets and multiwall carbon nanotubes on the structure and mechanical properties of poly(lactic acid) composites: a comparative study. Appl Sci 9:469
Proctor JE, Armada DAM (2017) An introduction to graphene and carbon nanotubes. Chapter 7: Raman spectra of graphene and single-walled carbon nanotubes (SWCNTs). Taylor & Francis Group, Boca Raton, Florida, pp 99–126
Hinkov I, Grand J, de la Chapelle ML, Farhat S, Scott CD, Nikolaev P, Pichot V, Launois P, Mevellec JY, Lefrant S (2004) Effect of temperature on carbon nanotube diameter and bundle arrangement: microscopic and macroscopic analysis. J Appl Phys 95:2029–2037
Chen J, Li Y, Huang L, Li C, Shi G (2015) High-yield preparation of graphene oxide from small graphite flakes via an improved Hummers method with a simple purification process. Carbon 81:826–834
Kovtun A, Jones D, Dell’Elce S, Treossi E, Liscio A, Palermo V (2019) Accurate chemical analysis of oxygenated graphene-based materials using X-ray photoelectron spectroscopy. Carbon 143:268–275
Rojas JV, Toro-Gonzalez M, Molina-Higgins MC, Castano CE (2016) Facile radiolytic synthesis of ruthenium nanoparticles on graphene oxide and carbon nanotubes. Mater Sci Eng, B 205:28–35
Thommes M, Kaneko K, Neimark AV, Olivier JP, Rodriguez-Reinoso F, Rouquerol J, Sing KSW (2015) Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC technical report). Pure Appl Chem 87:1051–1069
Peigney A, Laurent Ch, Flahaut E, Bacsa RR, Rousset A (2001) Specific surface area of carbon nanotubes and bundles of carbon nanotubes. Carbon 39:507–514
Theodorakopoulos GV, Arfanis MK, Sánchez Pérez JA, Agüera A, Cadena Aponte FX, Markellou E, Em G, Romanos P. Falaras (2023) Novel pilot-scale photocatalytic nanofiltration reactor for agricultural wastewater treatment. Membranes 13:202
Yu J, Grossiord N, Koning CE, Loos J (2007) Controlling the dispersion of multi-wall carbon nanotubes in aqueous surfactant solution. Carbon 45:618–623
Nguyen VS, Rouxel D, Hadji R, Vincent B, Fort Y (2011) Effect of ultrasonication and dispersion stability on the cluster size of alumina nanoscale particles in aqueous solutions. Ultrason Sonochem 18:382–388
Vasileiou AN, Theodorakopoulos GV, Karousos DS, Bouroushian M, Sapalidis AA, Favvas EP (2023) Nanocarbon-based mixed matrix Pebax-1657 flat sheet membranes for CO2/CH4 separation. Membranes 13:470
Alsharef JMA, Taha MR, Al-Mansob RA, Govindasamy P (2019) Evaluation of the dispersion stability of nanocarbons using zeta potential in distilled water. Nano Hybrids Compos 26:8–19
Riddick TM (1968) Control of colloid stability through zeta potential. Livingston Wynnewood, PA
Ran F, Lei W, Cui Y, Jiao J, Mao Y, Wang S, Wang S (2018) Size effect on oral absorption in polymer-functionalized mesoporous carbon nanoparticles. J Coll Inter Sci 511:57–66
Gudarzi MM, Moghadam MHM, Sharif F (2013) Spontaneous exfoliation of graphite oxide in polar aprotic solvents as the route to produce graphene oxide organic solvents liquid crystals. Carbon 64:403–415
Tantra R, Schulze P, Quincey P (2010) Effect of nanoparticle concentration on zeta-potential measurement results and reproducibility. Particuology 8:279–285
Acknowledgements
The authors would like to acknowledge the support of the project “CO2 separation by using mixed matrix, based on nano-carbon materials membranes (GG-CO2),” implemented under the “Action for the Strategic Cooperation between Greek and German Academia and Industry,” funded by the Operational Programme “Competitiveness, Entrepreneurship and Innovation” (NSRF 2014–2020) and co-financed by Greece and the European Union (European Regional Development Fund). This work was partially funded by “GRAPHITE” project (MIS 5185058), which has been co-funded by Greece and the European Union, through the framework program ATTIKI 2014–2020. The project “Intelligent Water Treatment for water preservation combined with simultaneous energy production and material recovery in energy intensive industries,” “IntelWATT,” funded from the European Union’s Horizon 2020 Research and Innovation Programme under grant agreement No 958454 is also acknowledged for the partial financial support. Diener Plasma Systems (German) and Surface Treat (Czech Republic) companies are acknowledged for the nanomaterials treatment. We also like to thank Prof. S. Hüttner and Felix Baier, Lehrstuhl für Experimantalphysik III at university of Bayreuth for the XPS measurements at the BPI KeyLab. The authors would also like to thank Prof. E. Efthimiadou (Dpt. of Chemistry, NKUA) for providing the Raman measurements and Dr. Elias Sakellis (NCSR “Demokritos”) and Prof. A. Mitropoulos (Dpt. of Chemistry, IHU) for their support regarding the obtaining of the TEM images.
Funding
Open access funding provided by HEAL-Link Greece.
Author information
Authors and Affiliations
Contributions
GVT contributed to data acquisition, data analysis, formal analysis and writing—original draft preparation. DSK was involved in investigation, formal analysis and reviewing and editing. JB, SF and RH contributed to data acquisition and reviewing and editing. AAS was involved in data acquisition and data analysis. FKK contributed to data acquisition, data analysis and reviewing and editing. TS was involved in funding acquisition and reviewing and editing. EPF contributed to conceptualization, supervision, data acquisition, data analysis, funding acquisition, writing—original draft preparation, and reviewing and editing.
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare that there is no conflict of interest in this work.
Ethical approval
There are no human subjects in this article, and informed consent is not applicable.
Additional information
Handling Editor: Annela M. Seddon.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Theodorakopoulos, G.V., Karousos, D.S., Benra, J. et al. Well-established carbon nanomaterials: modification, characterization and dispersion in different solvents. J Mater Sci 59, 3339–3362 (2024). https://doi.org/10.1007/s10853-024-09413-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-024-09413-x