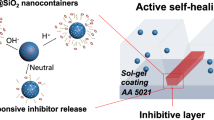
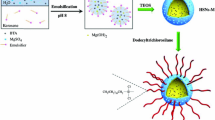
Abstract
Supramolecular silica systems, incorporating two different corrosion inhibitors (1H-Benzotriazole, BTA and 5-Phenyl-1H-tetrazole, PT), are successfully obtained via one-stage syntheses by self-assembly and characterised with a multi-analytical approach. Two different surfactants, namely cetyltrimethylammonium bromide (CTAB) or dodecylamine (DDA), are used both as templates and to promote synergistic corrosion inhibition in future application in smart and eco-sustainable coatings for metal substrates. The effect of the two different soft templates and the influence of a co-solvent (ethanol or methanol) on the morphology and the hierarchical porosity structure of the silica systems are investigated by electron microscopy techniques (SEM and TEM) and by nitrogen physisorption (BET/BJH). The confinement of both BTA and PT in mesoporous silica nanoparticles is qualitatively and quantitatively characterised by micro-Raman spectroscopy and thermal analyses. All synthesised composite samples show monodispersed nanoparticles (size in the range 50–500 nm). In the presence of CTAB soft template, spherical nanoparticle with non-intersecting longitudinal porosity is obtained and sphere-to-rod transition with chiral growth of particles is observed after the inhibitor encapsulation. On the contrary, by using DDA soft template, the symmetrical spherical shape of nanoparticles is retained when inhibitor is encapsulated, although with different diameter sizes. Radiant cylindrical-to-conical porosity is observed, depending on the solvent/co-solvent total volume and not influenced by the inhibitor. All supramolecular silica systems are characterised by a high loading capacity (30–40%) including both surfactants and azole compounds.
Graphical abstract







Copyright 2008 American Chemical Society








Similar content being viewed by others
Data and code availability
All data are available by contacting the corresponding author: antonella.privitera@uniroma3.it.
References
Liu T, Ma L, Wang X et al (2021) Self-healing corrosion protective coatings based on micro/nanocarriers: a review. Corros Commun 1:18–25. https://doi.org/10.1016/j.corcom.2021.05.004
Koch G (2017) Cost of corrosion. In: Trends in oil and gas corrosion research and technologies. Elsevier, pp 3–30
Tomashov ND, Chernova GP (1967) Passivity and protection of metals against corrosion. Springer, Boston
Nazeer AA, Madkour M (2018) Potential use of smart coatings for corrosion protection of metals and alloys: a review. J Mol Liq 253:11–22. https://doi.org/10.1016/J.MOLLIQ.2018.01.027
Saji VS (2019) Supramolecular concepts and approaches in corrosion and biofouling prevention. Corros Rev 37:187–230. https://doi.org/10.1515/corrrev-2018-0105
Maia F, Tedim J, Lisenkov AD et al (2012) Silica nanocontainers for active corrosion protection. Nanoscale 4:1287. https://doi.org/10.1039/c2nr11536k
Habib S, Qureshi A, Shakoor RA et al (2022) Corrosion inhibition performance of polyolefin smart self-healing composite coatings modified with ZnO@β-Cyclodextrin hybrid particles. J Market Res 21:3371–3385. https://doi.org/10.1016/J.JMRT.2022.10.148
Zhang F, Ju P, Pan M et al (2018) Self-healing mechanisms in smart protective coatings: a review. Corros Sci 144:74–88. https://doi.org/10.1016/J.CORSCI.2018.08.005
Shchukin DG (2013) Container-based multifunctional self-healing polymer coatings. Polym Chem 4:4871. https://doi.org/10.1039/c3py00082f
Wei H, Wang Y, Guo J et al (2015) Advanced micro/nanocapsules for self-healing smart anticorrosion coatings. J Mater Chem A Mater 3:469–480. https://doi.org/10.1039/C4TA04791E
Fateh A, Aliofkhazraei M, Rezvanian AR (2020) Review of corrosive environments for copper and its corrosion inhibitors. Arab J Chem 13:481–544. https://doi.org/10.1016/J.ARABJC.2017.05.021
(2000) Health Council of the Netherlands, Dutch Expert Committee on Occupational Standards (DECOS). 1,2,3-Benzotriazole. The Hague
Stupnišek-Lisac E, Božić AL, Cafuk I (1998) Low-toxicity copper corrosion inhibitors. Corrosion 54:713–720. https://doi.org/10.5006/1.3284890
Lu J, WANG MM, WANG Q, et al (2018) Determination of benzotriazole and its derivatives in aqueous sample with air-assisted liquid-liquid microextraction followed by high-performance liquid chromatography. Chin J Anal Chem 46:e1817–e1825. https://doi.org/10.1016/S1872-2040(17)61082-X
Privitera A, Porcelli F, Paoloni D et al (2023) Chemical-physical characterisation of 5-Phenyl-1H-tetrazole inhibitive behaviour: a new non-toxic compound for a sustainable protection of Cu-alloys. J Appl Electrochem 53:2375–2395. https://doi.org/10.1007/s10800-023-01936-6
Al Kharafi FM, Ghayad IM, Abdullah RM (2012) Corrosion inhibition of copper in non-polluted and polluted sea water using 5-phenyl-1-H-tetrazole. Int J Electrochem Sci 7:3289–3298
El Ibrahimi B, Guo L (2021) Azole-based compounds as corrosion inhibitors for metallic materials. In: Azoles—synthesis, properties, applications and perspectives. IntechOpen
Olivieri F, Castaldo R, Cocca M et al (2021) Innovative silver-based capping system for mesoporous silica nanocarriers able to exploit a twofold anticorrosive mechanism in composite polymer coatings: tailoring benzotriazole release and capturing chloride ions. ACS Appl Mater Interfaces 13:12. https://doi.org/10.1021/acsami.1c15231
Falcón JM, Batista FF, Aoki IV (2014) Encapsulation of dodecylamine corrosion inhibitor on silica nanoparticles. Electrochim Acta 124:109–118. https://doi.org/10.1016/J.ELECTACTA.2013.06.114
Falcón JM, Otubo LM, Aoki IV (2016) Highly ordered mesoporous silica loaded with dodecylamine for smart anticorrosion coatings. Surf Coat Technol 303:319–329. https://doi.org/10.1016/J.SURFCOAT.2015.11.029
Ma H, Chen S, Yin B et al (2003) Impedance spectroscopic study of corrosion inhibition of copper by surfactants in the acidic solutions. Corros Sci 45:867–882. https://doi.org/10.1016/S0010-938X(02)00175-0
Marconi E, Luisetto I, Di Carlo G et al (2023) 3-APTES on dendritic fibrous mesoporous silica nanoparticles for the pH-controlled release of corrosion inhibitors. Nanomaterials 13:2543. https://doi.org/10.3390/nano13182543
Ma X, Xu L, Wang W et al (2017) Synthesis and characterisation of composite nanoparticles of mesoporous silica loaded with inhibitor for corrosion protection of Cu-Zn alloy. Corros Sci 120:139–147. https://doi.org/10.1016/j.corsci.2017.02.004
Olivieri F, Castaldo R, Cocca M et al (2021) Mesoporous silica nanoparticles as carriers of active agents for smart anticorrosive organic coatings: a critical review. Nanoscale 13:9091–9111. https://doi.org/10.1039/D1NR01899J
Zea C, Alcántara J, Barranco-García R et al (2018) Synthesis and characterization of hollow mesoporous silica nanoparticles for smart corrosion protection. Nanomaterials 8:478. https://doi.org/10.3390/nano8070478
Singh LP, Bhattacharyya SK, Kumar R et al (2014) Sol-Gel processing of silica nanoparticles and their applications. Adv Colloid Interface Sci 214:17–37. https://doi.org/10.1016/J.CIS.2014.10.007
Chen H, He J, Tang H, Yan C (2008) Porous silica nanocapsules and nanospheres: dynamic self-assembly synthesis and application in controlled release. Chem Mater 20:5894–5900. https://doi.org/10.1021/cm801411y
Chan AC, Bravo Cadena M, Townley HE et al (2017) Effective delivery of volatile biocides employing mesoporous silicates for treating biofilms. J R Soc Interface 14:20160650. https://doi.org/10.1098/rsif.2016.0650
Popat A, Liu J, Hu Q et al (2012) Adsorption and release of biocides with mesoporous silica nanoparticles. Nanoscale 4:970–975. https://doi.org/10.1039/C2NR11691J
Borisova D, Möhwald H, Shchukin DG (2011) Mesoporous silica nanoparticles for active corrosion protection. ACS Nano 5:1939–1946. https://doi.org/10.1021/nn102871v
Zheng Z, Huang X, Schenderlein M et al (2013) Self-healing and antifouling multifunctional coatings based on pH and sulfide ion sensitive nanocontainers. Adv Funct Mater 23:3307–3314. https://doi.org/10.1002/adfm.201203180
Privitera A, Ruggiero L, Venditti I et al (2022) One step nanoencapsulation of corrosion inhibitors for gradual release application. Mater Today Chem 24:100851. https://doi.org/10.1016/j.mtchem.2022.100851
Knezevic NZ, Mauriello Jimenez C, Albino M et al (2017) Synthesis and characterization of core-shell magnetic mesoporous silica and organosilica nanostructures. MRS Adv 2:1037–1045. https://doi.org/10.1557/adv.2017.69
Nooney RI, Thirunavukkarasu D, Chen Y et al (2002) Synthesis of nanoscale mesoporous silica spheres with controlled particle size. Chem Mater 14:4721–4728. https://doi.org/10.1021/cm0204371
Collins TJ (2007) ImageJ for microscopy. Biotechniques 43:S25–S30. https://doi.org/10.2144/000112517
Gregg SJ, Sing KSW, Salzberg HW (1967) Adsorption surface area and porosity. J Electrochem Soc 114:279C. https://doi.org/10.1149/1.2426447
Liu K, Ostadhassan M (2019) The impact of pore size distribution data presentation format on pore structure interpretation of shales. Adv Geo-Energy Res; 3:187–197. https://doi.org/10.26804/ager.2019.02.08
Thommes M, Kaneko K, Neimark AV et al (2015) Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl Chem 87:1051–1069. https://doi.org/10.1515/pac-2014-1117
Du X, He J (2011) Spherical silica micro/nanomaterials with hierarchical structures: Synthesis and applications. Nanoscale 3:3984. https://doi.org/10.1039/c1nr10660k
Ren D, Xu J, Chen N et al (2021) Controlled synthesis of mesoporous silica nanoparticles with tunable architectures via oil-water microemulsion assembly process. Colloids Surf A Physicochem Eng Asp 611:125773. https://doi.org/10.1016/J.COLSURFA.2020.125773
Feng J, Liu Y, Liu C et al (2020) The impact of ethanol and chlorobenzene in the structure regulation of dendritic mesoporous silica nanoparticles. Microporous Mesoporous Mater 307:110504. https://doi.org/10.1016/J.MICROMESO.2020.110504
Li Y, Bi L, Wang S et al (2010) Preparation of helical mesoporous ethylene–silica nanofibers with lamellar mesopores on the surfaces. Chem Commun 46:2680. https://doi.org/10.1039/b926593g
Li J, Du X, Zheng N et al (2016) Contribution of carboxyl modified chiral mesoporous silica nanoparticles in delivering doxorubicin hydrochloride in vitro: pH-response controlled release, enhanced drug cellular uptake and cytotoxicity. Colloids Surf B Biointerfaces 141:374–381. https://doi.org/10.1016/j.colsurfb.2016.02.009
Faul CFJ, Antonietti M (2003) Ionic self-assembly: facile synthesis of supramolecular materials. Adv Mater 15:673–683. https://doi.org/10.1002/adma.200300379
Faul CFJ (2014) Ionic self-assembly for functional hierarchical nanostructured materials. Acc Chem Res 47:3428–3438. https://doi.org/10.1021/ar500162a
Franke D, Vos M, Antonietti M et al (2006) Induced supramolecular chirality in nanostructured materials: ionic self-assembly of perylene-chiral surfactant complexes. Chem Mater 18:1839–1847. https://doi.org/10.1021/cm0525499
Grzelak J, Gázquez J, Grayston A et al (2022) Magnetic mesoporous silica nanorods loaded with ceria and functionalized with fluorophores for multimodal imaging. ACS Appl Nano Mater 5:2113–2125. https://doi.org/10.1021/acsanm.1c03837
Xu Y, Li B, Xiao L et al (2013) The sphere-to-rod transition of squaraine-embedded micelles: a self-assembly platform displays a distinct response to cysteine and homocysteine. Chem Commun 49:7732. https://doi.org/10.1039/c3cc43223h
Qiu H, Wang S, Zhang W et al (2008) Steric and temperature control of enantiopurity of chiral mesoporous silica. J Phys Chem C 112:1871–1877. https://doi.org/10.1021/jp709798q
Rahmani S, Durand JO, Charnay C et al (2017) Synthesis of mesoporous silica nanoparticles and nanorods: application to doxorubicin delivery. Solid State Sci 68:25–31. https://doi.org/10.1016/J.SOLIDSTATESCIENCES.2017.04.003
Sing KSW (1985) Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (recommendations 1984). Pure Appl Chem 57:603–619. https://doi.org/10.1351/pac198557040603
Reber MJ, Brühwiler D (2015) Bimodal mesoporous silica with bottleneck pores. Dalton Trans 44:17960–17967. https://doi.org/10.1039/C5DT03082J
Lai W, Yang S, Jiang Y et al (2020) Artefact peaks of pore size distributions caused by unclosed sorption isotherm and tensile strength effect. Adsorption 26:633–644. https://doi.org/10.1007/s10450-020-00228-1
Chen X, Wang S, Zhuang J et al (2004) Mesoporous silica-supported NiB amorphous alloy catalysts for selective hydrogenation of 2-ethylanthraquinone. J Catal 227:419–427. https://doi.org/10.1016/j.jcat.2004.08.002
Ruggiero L, Bartoli F, Fidanza MR et al (2020) Encapsulation of environmentally-friendly biocides in silica nanosystems for multifunctional coatings. Appl Surf Sci 514:145908. https://doi.org/10.1016/j.apsusc.2020.145908
Dendramis AL, Schwinn EW, Sperline RP (1983) A surface-enhanced Raman scattering study of CTAB adsorption on copper. Surf Sci 134:675–688. https://doi.org/10.1016/0039-6028(83)90065-1
da Costa AMA, Geraldes CFGC, Teixeira-Dias JJC (1982) Micellar aggregation of CTAB in water and chloroform solutions—a study by laser raman spectroscopy. J Colloid Interface Sci 86:254–259. https://doi.org/10.1016/0021-9797(82)90063-7
Yeung H, Chan H, Weaver MJ (1999) A Vibrational Structural Analysis of Benzotriazole Adsorption and Phase Film Formation on Copper Using Surface-Enhanced Raman Spectroscopy. https://doi.org/10.1021/la981724f
Billes F, Endrédi H, Keresztury G (2000) Vibrational spectroscopy of triazoles and tetrazole. J Mol Struct (Thoechem) 530:183–200. https://doi.org/10.1016/S0166-1280(00)00340-7
Klapötke TM, Mayer P, Stierstorfer J, Weigand JJ (2008) Bistetrazolylamines—synthesis and characterization. J Mater Chem 18:5248. https://doi.org/10.1039/b811273h
de Ferri L, Lorenzi A, Lottici PP (2016) OctTES/TEOS system for hybrid coatings: real-time monitoring of the hydrolysis and condensation by Raman spectroscopy. J Raman Spectrosc 47:699–705. https://doi.org/10.1002/jrs.4881
Ek S, Root A, Peussa M, Niinistö L (2001) Determination of the hydroxyl group content in silica by thermogravimetry and a comparison with 1H MAS NMR results. Thermochim Acta 379:201–212. https://doi.org/10.1016/S0040-6031(01)00618-9
Pereira da Silva B, Saji VS, Aoki IV (2022) Rapid and eco-friendly one-step synthesis of dodecylamine-encapsulated mesoporous silica nanocontainers. Microporous Mesoporous Mater 341:112109. https://doi.org/10.1016/J.MICROMESO.2022.112109
Voinescu AE, Kellermeier M, Carnerup AM et al (2007) Co-precipitation of silica and alkaline-earth carbonates using TEOS as silica source. J Cryst Growth 306:152–158. https://doi.org/10.1016/J.JCRYSGRO.2007.03.060
Bari AH, Jundale RB, Kulkarni AA (2020) Understanding the role of solvent properties on reaction kinetics for synthesis of silica nanoparticles. Chem Eng J 398:125427. https://doi.org/10.1016/J.CEJ.2020.125427
Bernards TNM, van Bommel MJ, Boonstra AH (1991) Hydrolysis-condensation processes of the tetra-alkoxysilanes TPOS, TEOS and TMOS in some alcoholic solvents. J Non Cryst Solids 134:1–13. https://doi.org/10.1016/0022-3093(91)90005-Q
Andreeva NP, Kazanskii LP, Selyaninov IA et al (2009) Adsorption of 5-phenyltetrazole on iron and its inhibition of the dissolution of low-carbon steel in a neutral solution. Prot Met Phys Chem Surf 45:806–811. https://doi.org/10.1134/S2070205109070107
Hong S, Shen S, Cheng D et al (2016) Drug Delivery High drug load, stable, manufacturable and bioavailable fenofibrate formulations in mesoporous silica: a comparison of spray drying versus solvent impregnation methods High drug load, stable, manufacturable and bioavailable fenofibrate formulations in mesoporous silica: a comparison of spray drying versus solvent impregnation methods. Drug Deliv 23:316–327. https://doi.org/10.3109/10717544.2014.913323
Seljak KB, Kocbek P, Gašperlin M (2020) Mesoporous silica nanoparticles as delivery carriers: An overview of drug loading techniques. J Drug Deliv Sci Technol 59:101906. https://doi.org/10.1016/J.JDDST.2020.101906
Lehto VP, Riikonen J (2014) Drug loading and characterization of porous silicon materials. Porous Silicon Biomed Appl. https://doi.org/10.1533/9780857097156.3.337
Xu JB, Cao YQ, Fang L, Hu JM (2018) A one-step preparation of inhibitor-loaded silica nanocontainers for self-healing coatings. Corros Sci 140:349–362. https://doi.org/10.1016/J.CORSCI.2018.05.030
Yang B, Zhou S, Zeng J, et al Super-assembled core-shell mesoporous silica-metal-phenolic network nanoparticles for combinatorial photothermal therapy and chemotherapy. https://doi.org/10.1007/s12274-020-2736-6
Acknowledgements
The authors are grateful to Dr. Cadia D’Ottavi of the Department of Chemical Sciences and Technologies, Tor Vergata University and to Dr. Sergio Lo Mastro of the Department of Science, Roma Tre university, for the technical support. The availability of the Interdepartmental Electron Microscopy facility (LIME) at Roma Tre University is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
AP: Conceptualisation, methodology, formal analysis, investigation, data curation, visualisation, roles/writing—original draft, writing—review and editing. ST: formal analysis, data curation and resources. UPL: formal analysis and data curation. LR: methodology. LD: formal analysis and data curation. EDB: resources. ART: formal analysis and data curation. MAR: project administration, supervision and writing—review and editing. AS: project administration, supervision and writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that there is no conflict of interest.
Ethical approval
Not applicable. This article does not contain experiments involving human tissue or any ethical issues.
Additional information
Handling Editor: Annela M. Seddon.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Privitera, A., Tuti, S., Pasqual Laverdura, U. et al. One-stage synthesis and characterisation of supramolecular silica systems incorporating corrosion inhibitors for gradual release applications. J Mater Sci 59, 2497–2521 (2024). https://doi.org/10.1007/s10853-023-09306-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-023-09306-5