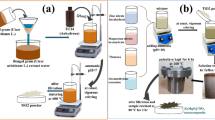
Abstract
The photothermal process exhibits the largest energy conversion efficiency among all solar energy utilization processes. In this study, a core–shell CoTiO3@MnO2 (CTM) composite photothermal catalyst was synthesized by supporting MnO2 on CoTiO3 by hydrothermal synthesis. Binding to peroxymonosulfate (PMS) heterogeneous catalyst removed tetracycline in wastewater. The new catalyst was characterized by FT-IR, UV–Vis, XRD, BET, SEM, TEM, and so on. Kinetic analysis showed that CTM activated PMS at a rate of 3.2 and 3.5 times higher than that of pure light and heat conditions, with complete degradation of tetracycline (TC). Since the outer MnO2 layer converted the absorbed solar energy into thermal energy, PMS was activated to generate active species while performing electron transfer, which resulted in a considerably improved catalytic efficiency. To prove the wide applicability of the catalyst, other pollutants were degraded, including bisphenol A (BPA), metronidazole (MNZ), and methyl orange (MO). The three pollutants were degraded within 40 min. Finally, our findings demonstrated the mechanism of the photothermal degradation process and provided a novel approach for environmental remediation using renewable solar energy.
Graphical Abstract
In this paper, the degradation efficiency of CoTiO3 was improved by loading MnO2 on CoTiO3. Binding to peroxymonosulfate (PMS) heterogeneous catalyst activation for the catalytic degradation of tetracycline. It was found by experiments that the core-shell structure of the composite material CoTiO3@MnO2 had better photothermal catalysis efficiency than photocatalysis and thermal catalysis.










Similar content being viewed by others
Data availability
Data will be made available on request.
References
Shen L, Chen J, Li N, He P, Li Z (2014) Rapid colorimetric sensing of tetracycline antibiotics with in situ growth of gold nanoparticles. Anal Chim Acta 839:83. https://doi.org/10.1016/j.aca.2014.05.021
Yan C, Yang Y, Zhou J et al (2013) Antibiotics in the surface water of the Yangtze Estuary: occurrence, distribution and risk assessment. Environ Pollut 175:22. https://doi.org/10.1016/j.envpol.2012.12.008
Zhan L, Xia Z, Xu Z, Xie B (2021) Study on the remediation of tetracycline antibiotics and roxarsone contaminated soil. Environ Pollut 271:116312. https://doi.org/10.1016/j.envpol.2020.116312
Tonetti MS (1998) Local delivery of terracycline: from concept to clinical application*. J Clin Periodontol 25:969. https://doi.org/10.1111/j.1600-051X.1998.tb02400.x
Kim H, Hong Y, Park J-e, Sharma VK, Cho S-i (2013) Sulfonamides and tetracyclines in livestock wastewater. Chemosphere 91:888. https://doi.org/10.1016/j.chemosphere.2013.02.027
Leong S, Li D, Hapgood K, Zhang X, Wang H (2016) Ni(OH)2 decorated rutile TiO2 for efficient removal of tetracycline from wastewater. Appl Catal B 198:224. https://doi.org/10.1016/j.apcatb.2016.05.043
Shi W, Guo F, Yuan S (2017) In situ synthesis of Z-scheme Ag3PO4/CuBi2O4 photocatalysts and enhanced photocatalytic performance for the degradation of tetracycline under visible light irradiation. Appl Catal B 209:720. https://doi.org/10.1016/j.apcatb.2017.03.048
Elimelech M, Phillip William A (2011) The future of seawater desalination: energy, technology, and the environment. Science 333:712. https://doi.org/10.1126/science.1200488
Ni G, Li G, Boriskina SV et al (2016) Steam generation under one sun enabled by a floating structure with thermal concentration. Nat Energy. https://doi.org/10.1038/nenergy.2016.126
Boddy PJ (1968) Oxygen Evolution on Semiconducting TiO2. J Electrochem Soc 115:199. https://doi.org/10.1149/1.2411080
Aslam M, Fazal DB, Ahmad F et al (2022) Photocatalytic degradation of recalcitrant pollutants of greywater. Catalysts. https://doi.org/10.3390/catal12050557
Turkten N, Natali Sora I, Tomruk A, Bekbolet M (2018) photocatalytic degradation of humic acids using LaFeO3. Catalysts. https://doi.org/10.3390/catal8120630
Fujishima A, Honda K (1972) Electrochemical photolysis of water at a semiconductor electrode. Nature 238:37. https://doi.org/10.1038/238037a0
Chen R, Jalili Z, Tayebee R (2021) UV-visible light-induced photochemical synthesis of benzimidazoles by coomassie brilliant blue coated on W-ZnO@NH2 nanoparticles. RSC Adv 11:16359. https://doi.org/10.1039/D0RA10843J
Jarrahi M, Tayebee R, Maleki B, Salimi A (2021) One-pot multicomponent green LED photoinduced synthesis of chromeno[4,3-b]chromenes catalyzed by a new nanophotocatalyst histaminium tetrachlorozincate. RSC Adv 11:19723. https://doi.org/10.1039/D1RA00189B
Li B, Tayebee R, Esmaeili E, Namaghi MS, Maleki B (2020) Selective photocatalytic oxidation of aromatic alcohols to aldehydes with air by magnetic WO3ZnO/Fe3O4. in situ photochemical synthesis of 2-substituted benzimidazoles. RSC Adv 10:40725. https://doi.org/10.1039/D0RA08403D
Mohammadzadeh Kakhki R, Tayebee R, Ahsani F (2017) New and highly efficient Ag doped ZnO visible nano photocatalyst for removing of methylene blue. J Mater Sci: Mater Electron 28(8):5941–5952. https://doi.org/10.1007/s10854-016-6268-5
Parvizi E, Tayebee R, Koushki E (2019) Mg-doped ZnO and Zn-doped MgO semiconductor nanoparticles; synthesis and catalytic optical and electro-optical characterization. Semiconductors 53:1769. https://doi.org/10.1134/S1063782619130141
Parvizi E, Tayebee R, Koushki E et al (2019) Photocatalytic efficacy of supported tetrazine on MgZnO nanoparticles for the heterogeneous photodegradation of methylene blue and ciprofloxacin. RSC Adv 9:23818. https://doi.org/10.1039/C9RA04702F
Tayebee R, Esmaeili E, Maleki B, Khoshniat A, Chahkandi M, Mollania N (2020) Photodegradation of methylene blue and some emerging pharmaceutical micropollutants with an aqueous suspension of WZnO-NH2@H3PW12O40 nanocomposite. J Mol Liq 317:113928. https://doi.org/10.1016/j.molliq.2020.113928
Wei Z, Abbaspour S, Tayebee R (2021) Nickel Nanoparticles originated from cressa leaf extract in the preparation of a novel melem@Ni-HPA photocatalyst for the synthesis of some chromenes and a preliminary MTT assay on the anticancer activity of the nanocomposite. Polycycl Aromat Compd. https://doi.org/10.1080/10406638.2021.2019063
Ramanavicius S, Ramanavicius A (2020) Insights in the application of stoichiometric and non-stoichiometric titanium oxides for the design of sensors for the determination of gases and VOCs (TiO2−x and TinO2n−1 vs. TiO2). Sensors 20:6833. https://doi.org/10.3390/s20236833
Sayegh S, Abid M, Tanos F et al (2022) N-doped TiO2 nanotubes synthesized by atomic layer deposition for acetaminophen degradation. Colloids Surf A 655:130213. https://doi.org/10.1016/j.colsurfa.2022.130213
Du Z, Cui C, Zhang S et al (2020) Visible-light-driven photocatalytic degradation of rhodamine B using Bi2WO6/GO deposited on polyethylene terephthalate fabric. JLSE 2:16. https://doi.org/10.1186/s42825-020-00029-w
Bao Y, Guo R, Gao M, Kang Q, Ma J (2021) Morphology control of 3D hierarchical urchin-like hollow SiO2@TiO2 spheres for photocatalytic degradation: Influence of calcination temperature. J Alloys Compd 853:157202. https://doi.org/10.1016/j.jallcom.2020.157202
Yu R, Lan T, Jiang J, Peng H, Liang R, Liu G (2020) Facile fabrication of functional cellulose paper with high-capacity immobilization of Ag nanoparticles for catalytic applications for tannery wastewater. JLSE 2:6. https://doi.org/10.1186/s42825-020-00019-y
Ghoussoub M, Xia M, Duchesne PN, Segal D, Ozin G (2019) Principles of photothermal gas-phase heterogeneous CO2 catalysis. Energy Environ Sci 12:1122. https://doi.org/10.1039/C8EE02790K
Zhu L, Gao M, Peh CKN, Ho GW (2018) Solar-driven photothermal nanostructured materials designs and prerequisites for evaporation and catalysis applications. Mater Horiz 5:323. https://doi.org/10.1039/c7mh01064h
Ma R, Sun J, Li DH, Wei JJ (2020) Review of synergistic photo-thermo-catalysis: mechanisms, materials and applications. Int J Hydrogen Energy 45:30288. https://doi.org/10.1016/j.ijhydene.2020.08.127
Xie W, Li Y, Shi W et al (2012) Novel effect of significant enhancement of gas-phase photocatalytic efficiency for nano ZnO. Chem Eng J 213:218. https://doi.org/10.1016/j.cej.2012.10.004
Gu Y, Jiao Y, Zhou X, Wu A, Buhe B, Fu H (2018) Strongly coupled Ag/TiO2 heterojunctions for effective and stable photothermal catalytic reduction of 4-nitrophenol. Nano Res 11:126. https://doi.org/10.1007/s12274-017-1612-5
Lai B-H, Lin Y-R, Chen D-H (2013) Fabrication of LaB6@SiO2/Au composite nanoparticles as a catalyst with near infrared photothermally enhanced activity. Chem Eng J 223:418. https://doi.org/10.1016/j.cej.2013.02.109
Christopher P, Xin H, Linic S (2011) Visible-light-enhanced catalytic oxidation reactions on plasmonic silver nanostructures. Nat Chem 3:467. https://doi.org/10.1038/nchem.1032
Lin L, Wang K, Yang K, Chen X, Fu X, Dai W (2017) The visible-light-assisted thermocatalytic methanation of CO2 over Ru/TiO(2–x)Nx. Appl Catal B 204:440. https://doi.org/10.1016/j.apcatb.2016.11.054
Liu F, Zeng M, Li Y, Yang Y, Mao M, Zhao X (2016) UV–vis–infrared light driven thermocatalytic activity of octahedral layered birnessite nanoflowers enhanced by a novel photoactivation. Adv Funct Mater 26:4518. https://doi.org/10.1002/adfm.201601046
Zeng M, Li Y, Mao M, Bai J, Ren L, Zhao X (2015) Synergetic effect between photocatalysis on TiO2 and thermocatalysis on CeO2 for gas-phase oxidation of benzene on TiO2/CeO2 nanocomposites. ACS Catal 5:3278. https://doi.org/10.1021/acscatal.5b00292
Low J, Yu J, Jaroniec M, Wageh S, Al-Ghamdi AA (2017) Heterojunction photocatalysts. Adv Mater 29:1601694. https://doi.org/10.1002/adma.201601694
Wang Y, Zhao Y, Liu J et al (2020) Manganese oxide modified nickel catalysts for photothermal co hydrogenation to light olefins. Adv Mater 10:1902860. https://doi.org/10.1002/aenm.201902860
Yuan R, Jiang Z, Wang Z et al (2020) Hierarchical MnO2 nanoflowers blooming on 3D nickel foam: a novel micro-macro catalyst for peroxymonosulfate activation. J Colloid Interface Sci 571:142. https://doi.org/10.1016/j.jcis.2020.03.041
Khalid MU, Warsi MF, Shakir I et al (2020) Al3+/Ag1+ induced phase transformation of MnO2 nanoparticles from α to β and their enhanced electrical and photocatalytic properties. Ceram Int 46:9913. https://doi.org/10.1016/j.ceramint.2020.01.143
Liang S, Teng F, Bulgan G, Zong R, Zhu Y (2008) Effect of phase structure of MnO2 nanorod catalyst on the activity for CO oxidation. J Phys Chem C 112:5307. https://doi.org/10.1021/jp0774995
Warshagha MZA, Muneer M (2022) Direct Z-scheme AgBr/β-MnO2 photocatalysts for highly efficient photocatalytic and anticancer activity. ACS Omega 7:30171. https://doi.org/10.1021/acsomega.2c03260
Chengjun R, Tao Z, Guoqiang C, Yaoqiang C, Maochu G (2006) Thermo-photocatalytic degradation of gas-phase benzene over Pt-TiO2/CeO2-MnO2 composite catalyst. Chin J Catal 27:1048
Ren C, Zhou L, Duan Y, Chen Y (2012) Synergetic effect of thermo-photocatalytic oxidation of benzene on Pt-TiO2/Ce-MnOx. J Rare Earths 30:1106. https://doi.org/10.1016/S1002-0721(12)60188-4
Xia D, Liu H, Xu B et al (2019) Single Ag atom engineered 3D-MnO2 porous hollow microspheres for rapid photothermocatalytic inactivation of E coli under solar light. Appl Catal B 245:177. https://doi.org/10.1016/j.apcatb.2018.12.056
Yang Y, Li Y, Zeng M et al (2018) UV–vis-infrared light-driven photothermocatalytic abatement of CO on Cu doped ramsdellite MnO2 nanosheets enhanced by a photoactivation effect. Appl Catal B 224:751. https://doi.org/10.1016/j.apcatb.2017.11.017
Yang Y, Wu S, Li Y, Zhang Q, Zhao X (2019) Efficient UV-vis-IR photothermocatalytic selective ethanol oxidation on MnOx/TiO2 nanocomposite significantly enhanced by a novel photoactivation. J Mater Chem A 8:1254–1264. https://doi.org/10.1039/C9TA12531K
Yin RY, Sun PF, Cheng LJ et al (2022) A three-dimensional melamine sponge modified with MnOx mixed graphitic carbon nitride for photothermal catalysis of formaldehyde. Molecules 27(16):5216. https://doi.org/10.3390/molecules27165216
Zheng Y, Wang W, Jiang D, Zhang L (2016) Amorphous MnOx modified Co3O4 for formaldehyde oxidation: improved low-temperature catalytic and photothermocatalytic activity. Chem Eng J 284:21. https://doi.org/10.1016/j.cej.2015.08.137
Eslami A, Hashemi M, Ghanbari F (2018) Degradation of 4-chlorophenol using catalyzed peroxymonosulfate with nano-MnO2/UV irradiation: toxicity assessment and evaluation for industrial wastewater treatment. J Clean Prod 195:1389. https://doi.org/10.1016/j.jclepro.2018.05.137
Huang Y, Tian X, Nie Y, Yang C, Wang Y (2018) Enhanced peroxymonosulfate activation for phenol degradation over MnO2 at pH 3.5–9.0 via Cu(II) substitution. J Hazard Mater 360:303. https://doi.org/10.1016/j.jhazmat.2018.08.028
Ndayiragije S, Zhang Y, Zhou Y et al (2022) Mechanochemically tailoring oxygen vacancies of MnO2 for efficient degradation of tetrabromobisphenol A with peroxymonosulfate. Appl Catal B 307:121168. https://doi.org/10.1016/j.apcatb.2022.121168
Wang G, Efstratiou A, Adjou Moumouni PF et al (2016) Primary Babesia rodhaini infection followed by recovery confers protective immunity against B. rodhaini reinfection and Babesia microti challenge infection in mice. Exp Parasitol 169:6. https://doi.org/10.1016/j.exppara.2016.07.003
Shi L, He Y, Wang X, Hu Y (2018) Recyclable photo-thermal conversion and purification systems via Fe3O4@TiO2 nanoparticles. Energy Convers Manag 171:272. https://doi.org/10.1016/j.enconman.2018.05.106
Hu F, Nan H, Wang M et al (2021) Construction of core-shell BaFe12O19@MnO2 composite for effectively enhancing microwave absorption performance. Ceram Int 47:16579. https://doi.org/10.1016/j.ceramint.2021.02.229
Zhang T, Guo Y, Li C et al (2020) The effect of LaFeO3@MnO2 on the thermal behavior of energetic compounds: an efficient catalyst with core-shell structure. Adv Powder Technol 31:4510. https://doi.org/10.1016/j.apt.2020.09.027
Fathy NA, El-Shafey SE, El-Shafey OI (2017) Synthesis of a novel MnO2@carbon nanotubes-graphene hybrid catalyst (MnO2@CNT-G) for catalytic oxidation of basic red 18 dye (BR18). J Water Process 17:95. https://doi.org/10.1016/j.jwpe.2017.03.010
Ye R, Fang H, Zheng Y-Z, Li N, Wang Y, Tao X (2016) Fabrication of CoTiO3/g-C3N4 Hybrid photocatalysts with enhanced H2 evolution: Z-scheme photocatalytic mechanism insight. ACS Appl Mater Interfaces 8:13879. https://doi.org/10.1021/acsami.6b01850
Chen JH, Huang TB, Wu X, Landheer D, Lei TF, Chao TS (2007) Performance improvement of CoTiO3 High-k dielectrics with nitrogen incorporation. J Electrochem Soc 154:G18. https://doi.org/10.1149/1.2388733
Fu J, Wang C, Feng Z, Zhang R (2018) Ultralong α-MnO2 nanowires capable of catalytically degrading methylene blue at low temperature. Catal Lett 148:2822. https://doi.org/10.1007/s10562-018-2454-9
Kumar S, Saeed G, Kim NH, Lee JH (2018) Hierarchical nanohoneycomb-like CoMoO4–MnO2 core–shell and Fe2O3 nanosheet arrays on 3D graphene foam with excellent supercapacitive performance. J Mater Chem A 6:7182. https://doi.org/10.1039/C8TA00889B
Goyal A, Sharma R, Bansal S, Tikoo KB, Kumar V, Singhal S (2018) Functionalized core-shell nanostructures with inherent magnetic character: outperforming candidates for the activation of PMS. Adv Powder Technol 29:245. https://doi.org/10.1016/j.apt.2017.11.008
Li X, Hou T, Yan L, Shan L, Meng X, Zhao Y (2020) Efficient degradation of tetracycline by CoFeLa-layered double hydroxides catalyzed peroxymonosulfate: synergistic effect of radical and nonradical pathways. J Hazard Mater 398:122884. https://doi.org/10.1016/j.jhazmat.2020.122884
Wang J, Wang S (2020) Reactive species in advanced oxidation processes: formation, identification and reaction mechanism. Chem Eng J 401:126158. https://doi.org/10.1016/j.cej.2020.126158
Wang H, Gao Q, Li H, Han B, Xia K, Zhou C (2019) One-pot synthesis of a novel hierarchical Co(II)-doped TiO2 nanostructure: toward highly active and durable catalyst of peroxymonosulfate activation for degradation of antibiotics and other organic pollutants. Chem Eng J 368:377. https://doi.org/10.1016/j.cej.2019.02.124
Xu LJ, Chu W, Gan L (2015) Environmental application of graphene-based CoFe2O4 as an activator of peroxymonosulfate for the degradation of a plasticizer. Chem Eng J 263:435. https://doi.org/10.1016/j.cej.2014.11.065
Saputra E, Muhammad S, Sun H, Ang HM, Tadé MO, Wang S (2013) Different crystallographic one-dimensional MnO2 nanomaterials and their superior performance in catalytic phenol degradation. Environ Sci Technol 47:5882. https://doi.org/10.1021/es400878c
Acknowledgements
This work was supported by the Hainan Provincial Natural Science Foundation of China (Grant Nos. 421RC483, 2019RC141, and ZDYF2021GXJS209); the National Natural Science Foundation of China (Grant Nos. 52161030 and 51901059); and the Foundation of State Key Laboratory of Marine Resource Utilization in South China Sea (Hainan University) (Grant No. MRUKF2021031).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Handling Editor: Pedro Camargo.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, X., Wei, S., Ma, X. et al. Core–shell CoTiO3@MnO2 heterostructure for the photothermal degradation of tetracycline. J Mater Sci 58, 3551–3567 (2023). https://doi.org/10.1007/s10853-023-08180-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-023-08180-5