Abstract
Polymer materials, not described in the literature so far, were obtained in the process of UV polymerization between methacrylic monomer obtained on the basis of natural, terpene alcohol: citronellyl methacrylate and benzyl methacrylate with different composition. The structures of the novel, environmentally friendly copolymers were confirmed by the FTIR and 13C CPMAS/NMR spectra. The copolymers containing more than 50% mass of citronellyl methacrylate were characterized by a high conversion of the double bonds determined on the basis of the FTIR and NMR spectra (95–96% and 92–94%, respectively). The novel materials were highly resistant to polar and non-polar solvents and the chemical stability. The glass transition temperature was from 15.8 to 19.9 °C which confirms that the obtained materials are elastomers at room temperature. Their thermal stability depended on their composition. It was from 185 to 205 °C (inert conditions) and from 149 to 214 °C (oxidizing conditions). TG/FTIR/QMS studies confirmed that their decomposition took place mainly as a depolymerization process combined with a subsequent breaking of the bonds in the resulting monomer/s at higher temperatures, which led to the formation of the gases with lower molecular masses. The main decomposition products emitted in an inert atmosphere were benzyl methacrylate, citronellyl methacrylate, 2-methylpropenal, citronellal and higher molecular mass compounds formed as a result of radical reactions between intermediate volatile products. In turn, under oxidizing conditions, as volatiles, benzyl methacrylate, citronellyl methacrylate, 2-methylpropenal, citronellal and small amounts of inorganic gases (CO, CO2, H2O) as a result of depolymerization and some combustion processes of the residues were indicated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Poly(methacrylates) are polymers, most often obtained in the photopolymerization processes and radical processes using suitable type initiators. This group of the polymeric materials is widely used in the industry as elements and components of many blends and compositions. The use of poly(methacrylates) is known, inter alia, in the cosmetics, optics, biomedicine, dentistry, nanotechnology, etc., due to their great properties. Among these properties, one should mention their reasonable resistance to chemicals, high impact strength, lightness, high resistance to sunshine and weather exposure, shatter and scratch resistances, good thermal resistance, compatibility with human tissue, wide range of glass transition temperature, etc. [1,2,3,4,5,6,7,8].
However, despite these good and excellent properties, research on the search for novel, more environmentally friendly methacrylic polymers with modified and/or improved properties for applications in the industry is still being carried out. The synthesis of such materials can take place in the processes of the chemical modification of natural polymers such as polysaccharides, proteins, biopolyesters, with the use of commercially available or new acrylic and methacrylic monomers [9,10,11,12,13,14,15,16,17,18] or in the processes of the polymerization of acrylic and methacrylic monomers prepared from natural compounds.
So far, it is known to use isosorbide to obtain acetylated acrylic and methacrylic isosorbide monomers by the tandem esterification with acetic anhydride and methacrylic anhydride [19,20,21,22] or syringyl methacrylate, creosyl methacrylate [23,24,25], vanillin methacrylate [24,25,26] to prepare bio-based polymeric materials for high-temperature applications. The use of new alkyl lactate monomers (methyl, ethyl, n-propyl and n-butyl lactate acrylates) to the preparation of bio-based acrylates is also known [27,28,29,30,31]. The synthesis of fatty acid-based meth(acrylate) monomers prepared in the esterification process of fatty alcohols with different chain lengths and meth(acrylic) acids or meth(acryloyl chlorides) or in the transesterification process of fatty alcohols with different chain lengths and meth(acrylates), e.g., methyl methacrylate is also described [32]. The possible application of new (meth)acrylates synthesized on the basis of 4-(4-hydroxyphenyl)butan-2-one extracted from the bark of Betula Pendula and treated with sodium hydroxide solution and meth(acryloyl chloride) in order to prepare novel polymeric materials is also reported [33]. In addition, the resin formulations based on natural phenolic acrylates containing a structural diacrylate synthesized by a dimerization of eugenol with a dithiol through the radical thiol-ene “click” reaction, a mono-methacrylate diluent (guaiacol methacrylate) and a methacrylate cross-linker (vanillyl alcohol methacrylate) have been tested for their use as more environmentally friendly materials for stereolitography 3D printing [34]. Also, the studies on the use of the new methacrylate monomer: eugenyl-2-hydroxypropyl methacrylate for the preparation of copolymers with methyl methacrylate are described [35]. Moreover, terpenes and terpenoids are very interesting group of compounds, due to the large variety of their structures which makes them interesting compounds for the synthesis of the new terpene bio-based polymers. The preparation of more environmentally friendly polymers based on pinene acrylate and methacrylate, limonene acrylate and methacrylate, carvone acrylate and methacrylate, menthyl acrylate and methacrylate, sorbrerol acrylate and methacrylate or tetrahydrogeraniol acrylate to obtain, among others, more environmentally friendly well-defined polymers and precise di- and multiblock copolymer architectures, novel green hydrophilic and amphiphilic terpene‐based polymers or high chemical and solvent-resistant, branched terpene methacrylate homopolymers from acrylate and methacrylate monomers prepared from natural terpene alcohols such as geraniol, nerol and citronellol and meth(acryloyl) chlorides is known, so far [36,37,38,39,40,41,42,43,44].
Of course, the above examples of meth (acrylate) monomers from natural compounds do not exhaust the list of monomers that can be used to synthesize more environmentally friendly polymeric materials compared to the petroleum-based polymers. The given examples only illustrate the recent trend to searching for polymeric materials with a more positive impact on the environment than traditional polymers.
In this paper, the use of methacrylate monomer synthesized in the amine catalyzed esterification process of natural, terpene alcohol: citronellol and methacryloyl chloride to the preparation of environmentally friendly copolymers with commercially available aromatic methacrylate monomer: benzyl methacrylate was proposed. The copolymers were obtained by the UV polymerization using different mass% of methacrylate monomers and 2,2,-dimethoxy-1,2-diphenylethan-1-one as an initiator. The influence of the mass% of methacrylate monomers on the double bond conversion (DC), glass transition temperature (Tg), solvent resistance, chemical resistance, thermal stability and the decomposition course in an inert and oxidizing atmosphere of the novel poly(citronellyl methacrylate)-co-poly(benzyl methacrylate) copolymers was presented and discussed.
Materials and methods
Materials
Citronellyl methacrylate was synthesized and purified according to the method described elsewhere [45,46,47]. Benzyl methacrylate was from Sigma-Aldrich (96%). Irgacure 651 (2,2,-dimethoxy-1,2-diphenylethan-1-one), methanol, chloroform, hexane, toluene, butanol and silica gel were from Merck. Sodium carbonate, magnesium sulfate, sodium hydroxide, carbon tetrachloride, hydrochloric acid, magnesium sulfate, buffers solutions (pH 4, 7, 10) were from POCh, Gliwice, Poland.
UV polymerization
The UV polymerization of monomer mixtures containing different mass% of methacrylate monomers was carried out in the presence of Irgacure 651 (3.0 mas%). The prepared mixtures of monomers: citronellyl methacrylate (CitM) and benzyl methacrylate (BM), Table 1, were irradiated using a TL20W/05 SLV low pressure mercury lamps with 340–365 nm. The circle samples with a diameter of 30 mm and thickness of 5 mm were irradiated for 10 min at 25ºC. Then, all prepared films were conditioned at 60 ºC for 5 h and at 120 ºC for 3 h to complete the polymerization.
Characterization of copolymers
FTIR Spectroscopy
The FTIR spectroscopy was used to check and confirm the structure of the prepared polymeric materials. The FTIR spectra (KBr tablets) were gathered from 600 cm−1 to 4000 cm−1 with 4 cm−1 resolution and 62 scans with the use of the FTIR Tensor 27 instrument (Bruker, Germany).
The conversion degree (DC) of the C = C bonds in methacrylate monomers after the UV polymerization and after post-polymerization was estimated based on the FTIR spectra. The DC was evaluated by the comparison of the area of the C = C stretching vibrations bands at 1635–1637 cm−1 (methacrylate bonds) and at 1674 cm−1 (ethylenic bonds) with the area of the C=O stretching vibration band at 1724 cm−1. The DC values for the obtained polymeric materials were calculated from the equation:
where R is the surface area of C=C absorption band/surface area of C=O absorption band [48, 49].
13 C CPMAS/NMR
The cross-polarization magic angle spinning (13C CPMAS/NMR) measurements were computed on a Bruker Avance 300 MSL instrument (Bruker, Germany) at resonance frequency of 75.5 MHz with the number of scans 2048 and the spin rate 7300 Hz.
Solubility Tests
The solubility of the obtained polymeric materials was determined by the gravimetric method at 25 °C. The solvents of different polarity such as water, methanol, butanol, toluene, hexane, carbon tetrachloride, chloroform, cyclohexane, dioxane, acetone, acetic acid, ethyl acetate, tetrahydrofuran, diethyl ether were chosen. The sample of ca. 0.2 g was immersed in the suitable solvent and kept over 6 months. Then, the samples were removed from the solvents, dried and weighed. The percentage change in the mass (ΔmS) is determined from the following equation:
where m1—the initial mass of the polymeric sample, m2—the final mass of the polymeric sample, ΔmS—the percentage change in mass (the solubility of the materials).
Chemical resistance
The chemical resistance of the obtained polymeric materials was evaluated in alkaline, buffers and acidic environments at 25 °C. As environments 1 M NaOH, buffer solutions with pH 4, 7 and 10 and 1 M HCl were used. The sample of ca. 0.2 g was placed in a suitable chemical environment and kept over 6 months. Then, the samples were removed from these solutions, carefully washed with distilled water, dried and weighed. The percentage change in the mass (ΔmR) was evaluated from equation [50]:
where m1—the initial mass of the polymeric sample, m2—the final mass of the polymeric sample, ΔmR—the percentage change in the mass (the chemical resistance of the polymeric material to a specific environment).
Glass transition temperature (T g )
The glass transition temperature (Tg) of the obtained polymeric materials was evaluated using a DSC method. The sample of ca. 10 mg was placed in an aluminum crucible with a pierced lid and heated from minus 120 °C to 150 °C in an argon atmosphere (a flow rate 40 mL/min). The heating rate was 10 K/min. Two DSC scans from minus 120 °C to 150 °C were performed. The Tg was evaluated from the second DSC scan.
Thermal properties (TG/DTG)
The thermal properties (TG/DTG) of the materials were determined with a use of a TG/DTG method. The STA 449 Jupiter F1 instrument (Netzsch, Germany) was used. The sample of ca. 10 mg was heated from 40 °C to 550 °C in an open Al2O3 crucible and with a heating rate 10 K/min. The TG/DTG analyses were performed in an inert atmosphere (a helium with a flow rate 40 mL/min) and in an oxidizing atmosphere (a synthetic air atmosphere with a flow rate 100 mL/min). The initial decomposition temperatures marked as temperatures where there is a mass loss of 5% (T5%), maximum decomposition temperatures (Tmax), mass loss in each decomposition stage (Δm) and residual masses (rm) were evaluated.
Simultaneous TG-FTIR-QMS analysis
The volatiles emitted during the heating of the polymeric materials were analyzed by a simultaneous TG-FTIR-QMS analysis. A STA 449 Jupiter F1 instrument (Netzsch, Germany) was connected on-line to the FTIR analyzer (FTIR TGA 585, Bruker, Germany) and the QMS analyzer (QMS 403 C Aëolos, Germany). The emitted volatiles were collected in both atmospheres: inert and oxidizing. The FTIR analyzer was connected on-line to a STA instrument through a Teflon tube 2 mm with a diameter and heated to 200 °C to avoid the condensation process of volatiles. The gaseous FTIR spectra were collected from 600 to 4000 cm−1 and 4 cm−1 resolution. The QMS analyzer was linked on-line to a STA instrument by a quartz capillary heated to 300 °C. The QMS was operated under electron ionization 70 eV. The QMS spectra were gathered from 10 to 170 m/z.
Results and discussion
FTIR Spectroscopy
The FTIR spectra for the tested polymeric materials are presented in Fig. 1. The FTIR spectra show all the absorption signals for the characteristic groups present in the structure of the copolymers. The following vibrations are observed from the FTIR spectra of the copolymers: the stretching vibrations of the CAr-H and = C–H (3014–3070 cm−1), the stretching vibrations of the C–H (2877–2966 cm−1), the stretching vibrations of the C = O (1724 cm−1), the stretching vibrations of C = C (1635–1637 cm−1 and 1674 cm−1 with a very low intensity for copolymers 2 and 3), the stretching vibrations of the CAr = CAr (1456–1496 cm−1), the deformation vibrations of the C-H (1353–1456 cm−1), the stretching vibrations of the C-O (1099–1272 cm−1) and the out of plane deformation vibrations of the CAr-H and = C–H (663–970 cm−1) as it is marked in Fig. 1. The FTIR results confirm that the two types of double bonds present in citronellyl methacrylate are involved in the UV polymerization process. The absorption bands responsible for the stretching vibrations for methacrylate C = C bonds are localized at 1635–1637 cm−1. In turn, the absorption bands for ethylenic C = C double bonds appeared at 1674 cm−1 [38]. If we look at the FTIR spectra for the copolymers, it can be seen that the absorption bands characteristics for two types of the C = C stretching vibrations have residual intensities, Fig. 1.
On the basis of the conversion degree (DC) calculations, it can be seen that the polymerization process and subsequent cross-linking process take place with high efficiency, especially for the copolymers with a certain composition. The DC values are in the range 95–96% for the copolymers containing from 50 to 80 mass% of citronellyl methacrylate (copolymers 2 and 3). In addition, after post-polymerization DC value increases by an average of 5–11%, Table 2. It confirms that the polymeric materials with a high degree of polymerization are obtained. This also indicates that both methacrylic and ethylenic C = C bonds participate in the polymerization process. This leads to the formation of branched, cross-linked materials [38, 51, 52]. However, for copolymer containing 20 mass% of citronellyl methacrylate (copolymer 1), the lower value of DC may be caused by a steric hindrance (benzyl rings), Table 2.
13 C CP/MAS NMR
The assignments for the chemical shifts in the 13C CP/MAS NMR for the studied polymeric materials are presented in Table 3. The presented results indicate that the peaks associated with the carbonyl in unreacted methacrylate groups for the tested copolymers are visible at 166 ppm. Their intensity is low, in particular for the copolymers 2 and 3, Fig. 2. However, the carbonyl in reacted methacrylate groups is present at 175–176 ppm. This confirms the results obtained from the FTIR analysis and proves high conversions of methacrylate double bonds during the UV polymerization and stabilization of these materials. In addition, low-intensity NMR signals for the carbons with unreacted ethylene bonds at 132 and 122 ppm are observed for the copolymers 2 and 3. The intensity of the carbons with unreacted ethylene bonds for copolymer 1 is higher as compared to this intensity for the copolymers 2 and 3. It confirms the lower reactivity of ethylenic double bonds compared to the reactivity of methacrylate bonds in the polymerization process. The % of the unreacted C = C double bonds is in agreement with the FTIR data. The % of unreacted C = C double bonds calculated from the 13C CP/MAS NMR spectra was 22% (copolymer 1), 6% (copolymer 2) and 8% (copolymer 3).
Based on the results of the FTIR spectroscopy and the cross-polarization magic angle spinning (13C CPMAS/NMR) measurements, the simplified structure of the copolymers is presented in Scheme 1.
Solubility Tests
The results from the solubility tests are placed in Tables 4 and 5. As it can be seen, the solubility of the copolymers and PCitM in both types of solvents (polar and non-polar) is very low. The solubility is below 1.8% for the samples kept in the solvents for 6 months. This is connected with its branched structure containing additional cross-links as it was confirmed based on the FTIR and 13C CP/MAS NMR analyses.
Chemical resistance
Table 6 contains the chemical resistance results. The tested polymeric materials are highly resistant to alkaline, buffer and acidic environments. The percentage change in the mass is below 1.6%. In addition, it was noticed that the samples have not changed their color or shape after chemical resistance tests. Before the tests, the samples were colorless or slightly yellowish.
The results confirm that the prepared copolymers can be applied as high chemical and solvent-resistant polymeric materials for the preparation of different parts and elements work in aggressive environments or as potential protective coatings.
Glass transition temperature (T g )
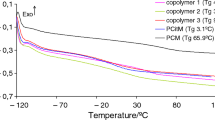
As tested using a DSC method, the glass transition temperature of the copolymers is slightly depended on the percentage of monomers used to obtain them. PCitM homopolymer is characterized by Tg of ca. 3.1 °C. In turn, PBM homopolymer has Tg of ca. 48.5 °C, while the copolymers show Tg of 15.8–19.9 °C, Fig. 3. The same Tg values for copolymers containing from 50 mass% to 80 mass% of citronellyl methacrylate also indicate the similar degree of polymerization and cross-linking, as it is confirmed based on the FTIR and 13C CP/MAS NMR analyses. However, a slightly higher Tg for the copolymer 1 (19.9 °C) with a lower DC value may be due to the higher content of aromatic rings in its structure. However, as can be seen from the results, the addition of citronellyl methacrylate to the copolymers causes a significant reduction in Tg compared to PBM and thus an increase in the flexibility of the materials at room temperature. Moreover, the copolymers show only one Tg indicating that random copolymers are obtained.
Thermal properties (TG/DTG) in inert atmosphere
The thermal stability of the tested copolymers is in the range of 185–205 °C, Fig. 4, Table 7. As it is visible, the thermal resistance of the novel polymeric materials is lower than the thermal stability of PCitM and PBM homopolymers. In addition, the values of T5% decrease with increasing citronellyl methacrylate monomer in the composition. The lower thermal stability of the copolymers compared to that of homopolymers may be due to the initiation of depolymerization at the ends of the chain for the copolymers at lower temperatures. Most likely, the addition of citronellyl methacrylate to the composition with benzyl methacrylate in this system reduces the activation energy and breaks the bonds at a lower temperature.
As can be seen from the presented graphs, the degradation of the copolymers in an inert atmosphere is at least two-stage process. The first decomposition stage is visible as a low intensity, “stretch” DTG peak from T5% to 265 °C with Tmax1 at 202–223 °C and the mass loss (Δm1) ranges from 10.5% (copolymer 1) to 22.9% (copolymer 3). The second decomposition stage is observed as a DTG peak of significant intensity from 265 °C to 470 °C with Tmax2 at 355–401 °C and the mass loss (Δm2) 89.5% (copolymer 1), 85.8% (copolymer 2) and 77.1% (copolymer 3) and with the rate of − 11.94%/min (copolymer 1), − 10.83%/min (copolymer 2) and − 9.46%/min (copolymer 3). Also, the decomposition of homopolymers (PCitM and PBM) runs also in at least two stages connecting with the breaking of the bonds in their structures, Fig. 4 and Table 7. The full decomposition of homopolymers is observed at 470 °C.
What's interesting is that the PCitM and PBM homopolymers decomposition in an inert atmosphere is described as an endothermic DSC peak. The presence of only the endothermic peak indicates the pyrolysis processes of the bonds present in the structure of homopolymers. While if we look at the DSC curves for the copolymers, we can observe the presence of an asymmetric, exothermic peak in an inert atmosphere. Its presence suggests the simultaneous pyrolysis reactions of the bonds present in the copolymer structure and the chemical reactions that may take place between gaseous decomposition products and/or residues formed during the decomposition of the tested materials.
Simultaneous TG-FTIR-QMS analysis in inert atmosphere
The gaseous 3D FTIR spectra and the gaseous FTIR spectra extracted at T5%, Tmax1 and Tmax2 are presented in Fig. 5. On the basis of the presented FTIR spectra, it can be seen that the emission of the mixture of volatiles is observed. In addition, the intensity of a gas emission decreases with increasing citronellyl methacrylate content in the copolymers. In principle, no gas emissions are observed at T5%, which may be due to their low concentration undetectable by the FTIR analyzer. The gradual increase in the temperature to Tmax1 causes that the FTIR spectra begin to show a signal characteristic for the stretching vibration of carbonyl group (at 1735–1776 cm−1) but still of very low intensity. However, increasing the heating temperature to Tmax2 causes a high intensity of the emitted gases. Among them, we can notice the presence of the absorption signals characteristic for unsaturated and aromatic fragments: the stretching vibrations of the = C–H and CAr = CAr at 3018 cm−1 and 3052 cm−1, the stretching vibrations of the C = C at 1621–1625 cm−1 and the out of plane deformation vibrations of the = C-H and CAr = CAr at 930–990 cm−1 and 692–798 cm−1, respectively. Additionally, the presence of the absorption bands is noticeable for compounds with the C = O and C–O groups in their structure: the stretching vibrations of the C = O at 1735 cm−1 and at 1780–1785 cm−1 and the stretching vibrations of the C–O at 1153–1297 cm−1 [53,54,55,56,57].
To more accurately confirm the structure of gases released during the decomposition of the copolymers, the QMS analysis was also performed, and the results are shown in Fig. 6. The QMS analysis indicates that one of the decomposition products is aromatic monomer: benzyl methacrylate. Its presence, among volatiles, is confirmed based on the following m/z ions in the QMS spectrum: 39, 41, 65, 69, 79, 90, 91, 107, 131 [58]. In addition, it should be noted that as the content of benzyl methacrylate in the copolymers decreases, the intensity of the aforementioned m/z ions decreases, which is correct and understandable. Apart from the emission of benzyl methacrylate, the emission of volatiles formed from citronellyl fragments is expected. Our previous research showed that the poly(citronellyl methacrylate) homopolymer decomposed to a monomer in inert conditions. It was confirmed by the presence in the FTIR spectrum of, inter alia, the stretching vibrations for the C = O group at 1736 cm−1 [38]. The FTIR spectra collected during the decomposition of the tested copolymers also show this absorption signal but this signal is asymmetrical with two maxima, one at 1733–1735 cm−1, and the other at 1776 cm−1. The presence of the absorption signals for the carbonyl group with two maxima and the presence of the exothermic DSC peak may indicate the formation of ester and the decomposition products with lower masses. Analyzing the QMS spectra, the presence of m/z ions characteristic for citronellyl methacrylate such as 50, 51, 52, 53, 54, 55, 56, 57, 58, 65, 68, 69, 70, 77, 79, 91, 92 [38, 58] and for the decomposition products of citronellyl methacrylate such as 2-methylpropenal (m/z 41, 70, 39, 42, 29, 38, 40, 27, 37, 69) and citronellal (m/z 41, 69, 55, 95, 43, 56, 67, 29, 39, 27) is noticed, Scheme 2. However, due to the presence of an exothermic peak on the DSC, it is highly probable that the formed unsaturated aldehydes can undergo polymerization under the test conditions to form compounds with higher molecular masses. The pyrolysis path of the tested copolymers evaluated based on the simultaneous TG-FTIR-QMS analysis is shown in Scheme 2.
Thermal properties (TG/DTG) in a synthetic air atmosphere
Figure 7 presents the TG/DTG/DSC curves obtained for the tested copolymers heated in synthetic air conditions. Generally, the tested copolymers show lower thermal resistance than homopolymers and copolymers tested in an inert atmosphere, except for the copolymer 1. Their thermal resistance ranges from 214 to 149 °C and depends on the mass% of monomers used to obtain them. The highest thermal stability is observed for the copolymer 1, which is obtained with the composition of the monomers: 80 mass% of benzyl methacrylate and 20 mass% of citronellyl methacrylate. As citronellyl methacrylate content of the compositions increases, the thermal resistance of the copolymers decreases, Table 8.
The oxidative decomposition of the copolymers run at least in two main stages, wherein the first decomposition stage is composed of at least two or three steps. The first decomposition stage is described as an asymmetric DTG signal from T5% to 450 °C with two or three maxima marked as Tmax1, Tmax1a and Tmax1b. The mass loss (Δm1) at this stage of the decomposition is significant. The mass loss is 98.1% with a rate of -10.42%/min (copolymer 1), 90.3% with a rate of -9.53%/min (copolymer 2) and 77.8% with a rate of -10.45%/min (copolymer 3) and depends on the nature of the copolymers. In turn, the second decomposition stage of the copolymers is described by a symmetric DTG peak visible from 450 to 580 °C and with Tmax2 from 486 to 510 °C. The mass loss (Δm2) is from 1.9 to 22.2% and depends on the composition of the copolymers. Moreover, in the case of homopolymers, mainly exothermic signals are observed in the DSC curves. It proves the chemical reactions taking place between the decomposition products of homopolymers and oxygen. What is interesting is that in the DSC curves for the tested copolymers, only the endothermic signals are present. It suggests mainly the cleavage of the bonds in the structure of the copolymers. This indicates a different course of the decomposition of homopolymers and copolymers in an oxidizing atmosphere. In the case of the copolymers, the presence of oxygen inhibits the formation of free radical intermediates. Therefore, mainly decomposition rather than chemical reactions takes place in the presence of oxygen.
Simultaneous TG-FTIR-QMS analysis in a synthetic air atmosphere
The FTIR gas spectra for the copolymers collected from T5% to Tmax1 show the presence of the following absorption bands: the stretching vibrations of the = C–H and CAr = CAr at 3025 cm−1 and 3058 cm−1, the stretching vibrations for the compounds with the C = O groups at 1733–1735 cm−1 with a low intensity band at 1774 cm−1 (copolymers 1 and 2) or 1785 cm−1 (copolymer 3), the stretching vibrations of the C = C at 1621–1623 cm−1, the deformation vibrations of the C-H at 1440–1370 cm−1, the stretching vibrations of the C–O at 1297–1051 cm−1 and the out of plane deformation vibrations of the = C–H and CAr = CAr at 912–1000 cm−1 and 690–800 cm−1, respectively [53,54,55]. The presence of these absorption bands may indicate the emission of ester fragments and a lower molecular mass compounds containing unsaturated bonds, aromatic rings and/or carbonyl groups in the structure. In addition, at this temperature range, a low emission of CO2 (2358–2333 cm−1) is also observed. At Tmax2, mainly the emission of CO2 and H2O is confirmed, Fig. 8 [56, 57, 59].
The QMS spectra collected at Tmax1, Tmax1a and Tmax2 for the selected copolymer (copolymer 2) are presented in Fig. 9. As it is well visible, the appearance of the following m/z ions: 14, 15, 16, 17, 18, 20, 27, 36, 39, 44 at Tmax1 is confirmed. However, at Tmax1a, the number of m/z ions is significantly increased, which is related to the release of the main decomposition products during heating. At Tmax1a, the presence of the m/z ions characteristic for the emission of benzyl methacrylate monomer is observed (m/z 39, 41, 65, 69, 79, 90, 91, 107, 131) [58]. In addition, the QMS spectra show the presence of m/z ions: 27, 29, 37, 38, 39, 40, 41, 42, 43, 50, 51, 52, 53, 54, 55, 56, 57, 58, 65, 67, 68, 69, 70, 77, 79, 91, 92 which are derived from decomposition products such as citronellyl methacrylate, citronellal and 2-methylpropenal. At Tmax2, mainly the emission of H2O (m/z 16, 17, 18), CO2 (m/z 44) and CO (m/z 28) is visible [58] which indicate on the combustion processes of the formed residues [60, 61].
Conclusions
The more environmentally friendly polymeric materials with a high conversion of the double bonds evaluated based on the FTIR and 13C CPMAS/NMR spectra and with an excellent solvent and chemical resistance were prepared in the UV polymerization of benzyl methacrylate monomer and methacrylate monomer synthesized in the esterification of natural, terpene alcohol (citronellol) and methacryloyl chloride. The DSC analysis proved the glass transition temperature of these copolymers in the range of 15.8–19.9 °C. The TG/DTG analysis indicated that the copolymers containing up to 50 mass% of citronellyl methacrylate were characterized by a thermal resistance above 200 ºC (inert conditions) and above 185 °C (oxidizing conditions). In addition, the FTIR and QMS analyses of the gaseous decomposition products confirmed that the decomposition of the copolymers was complex and included the depolymerization reactions and intermediate products decomposition reactions combined with possible radical reactions under inert and oxidizing conditions. This led to the emission of a mixture of the following gaseous products: benzyl methacrylate, citronellyl methacrylate, 2-methylpropenal, citronellal and a higher molecular mass compounds due to the possible radical reactions in an inert atmosphere. During the oxidation conditions, the formation of the same, major volatiles as in the inert atmosphere was observed. It suggested that the presence of oxygen did not affect the decomposition process of the tested copolymers. As additional gaseous decomposition products, only small amounts of CO, CO2 and H2O as a result of the combustion process of the residues were observed.
Summing up, these novel environmentally friendly poly(citronellyl methacrylate)-co-poly(benzyl methacrylate) copolymers due to their properties can be applied as the materials working in corrosive conditions, potential protective coatings or as materials for other uses in everyday life.
References
Li H, Wang H, Liu C, Huan X, Dong J, Li W, Xu K, Geng H, Guo X, Jia X, Yang Y (2022) PGMA-grafted MWCNTs: correlation between molecular weight of grafted PGMA and dispersion state of MWCNTs–PGMA in an epoxy matrix. J Mater Sci 57:7997–8015.
Kost J, Langer R (2012) Responsive polymeric delivery systems. Adv Drug Deliv Rev 64:327–341.
Yedewar PG, Wadhaj SM, Sawane YB, Banpurkar AG (2022) Polymethyl methacrylate (PMMA)/fluoropolymer bilayer: a promising dielectric for electrowetting applications. J Mater Sci 57:9018–9027.
Mishra S, Sen G (2011) Microwave initiated synthesis of polymethylmethacrylate graft guar (GG-g-PMA), characterizations and applications. Int J Biolog Macromol 48:688–694.
Lu Y, Yamago S (2020) Synthesis of structurally controlled, highly branched polymethacrylates by radical polymerization through the design of a monomer having hierarchical reactivity. Macromolecules 53:3209–3216.
Malcom PS Polymer Chemistry: An Introduction 3rd ed. Oxford University Press, Oxford, NY, pp. 167–176.
Gorge O (2004) Principles of polymerization, 4th edn. Wiley, NJ, pp 198–235.
Clarke A, LeBlanc E, Angelatos C, Russell K, Karim S, Wells LA (2022) the effects of surface chemistry on the accumulation of extracellular traps on poly(methyl methacrylate) and the implications on other immune cells. J Mater Sci 57:10299–10312.
Meimoun J, Wiatz V, Saint-Loup R, Parcq J, Favrelle A, Bonnet F, Zinck P (2018) Modification of starch by graft copolymerization. Starch 70:1600351.
Çankaya N (2016) Synthesis of graft copolymers onto starch and its semiconducting properties. Results Phys 6:538–542.
Noordergraaf IW, Fourie TK, Raffa P (2018) Free-radical graft polymerization onto starch as a tool to tune properties in relation to potential applications. A review Processes 6:31.
Kang H, Liu R, Huang Y (2015) Graft modification of cellulose: Methods, properties and applications. Polymer 70:A1–A16.
Kumar R, Sharma RK, Singh AP (2018) Grafted cellulose: a bio-based polymer for durable applications. Polym Bull 75:2213–2242.
Tanodekaew S, Prasitsilp M, Swasdison S, Thavornyutikarn B, Pothsree T, Pateepasen R (2014) Preparation of acrylic grafted chitin for wound dressing application. Biomaterials 25:1453–1460.
Samit A, Ray K (2021) Synthesis of chitosan grafted polymethyl methacrylate nanopolymers and its effect on polyvinyl chloride membrane for acetone recovery by pervaporation. Carbohydr Polym 258:117704.
Jin Z, Feng W, Zhu S, Sheardown H, Brash JL (2010) Protein-resistant polyurethane by sequential grafting of poly(2-hydroxyethyl methacrylate) and poly(oligo(ethylene glycol) methacrylate) via surface-initiated ATRP. J Biomed Mater Res A 15:1223–1232.
Yu L, Li C, Liu Y, Sun Y (2021) Protein adsorption to poly(2-aminoethyl methacrylate)-grafted Sepharose gel: Effects of chain length and charge density. J Chrom A 1638:461869.
Hazer B (1995) Grafting reactions onto polymer backbone with polymeric initiator. J Macromol Sci Part A 32:679–685.
Gallagher JJ, Hillmyer MA, Reineke TM (2015) Isosorbide-based polymethacrylates. CS Sustain Chem Eng 4:662–667.
Liu W, Xie T, Qiu R (2017) Biobased thermosets prepared from rigid isosorbide and flexible soybean oil derivatives. ACS Sustain Chem Eng 5:774–783.
Baek SS, Jang SH, Hwang SH (2017) Sustainable isosorbide-based transparent pressure-sensitive adhesives for optically clear adhesive and their adhesion performance. Polym Int 66:1834–1840.
Matt L, Parve J, Parve O, Pehk T, Pham TH, Liblikas I, Vares L, Jannasch P (2018) Enzymatic synthesis and polymerization of isosorbide-based monomethacrylates for high-Tg plastics. ACS Sustain Chem Eng 6:17382–17390.
Holmberg AL, Reno KH, Nguyen NA, Wool RP, Epps TH (2016) Syringyl methacrylate, a hardwood lignin-based monomer for high-Tg polymeric materials. ACS Macro Lett 5:574–578.
Epps TH III, Holmberg TH, Nicastro AL, Wang KH, Saha S, Li BS, Morris VDG, Melody A (2018) Bio-based polymers from raw lignocellulosic biomass. United States Patent Appl 201:90–144.
Bassett AW, Sweet KR, O-Dea RM, Honnig AE, Breyta CM, Reilly JH, La Scala JJ, Epps TH, Stanzione JF (2020) Dual-functional, aromatic, epoxy-methacrylate monomers from bio-based feedstocks and their respective epoxy-functional thermoplastics. J Polym Sci 58:673–682. https://doi.org/10.1002/pol.20190110.
Zhang C, Madbouly SA, Kessler MR (2015) Renewable polymers prepared from vanillin and its derivatives. Macromol Chem Phys 216:1816–1822.
Srikanthan V, Pitois O, Coussot P, Le Droumaguet B, Grande D (2021) Wood-mimicking bio-based biporous polymeric materials with anisotropic tubular macropores. Polymers 13:2692.
Xu Y, Odelius K, Hakkarainen M (2020) Photocurable, thermally reprocessable, and chemically recyclable vanillin-based imine thermosets. ACS Sustain Chem Eng 8:17272–17279.
Hasegawa S, Azuma M, Takahashi K (2008) Stabilization of enzyme activity during the esterification of lactic acid in hydrophobic ethers and ketones as reaction media that are miscible with lactic acid despite their high hydrophobicity. Enzyme Microb Technol 43:309–316.
Gao C, Ma C, Xu P (2011) Biotechnological routes based on lactic acid production from biomass. Biotechnol Adv 29:930–939.
Purushothaman M, Krishnan PSG, Nayak SK (2016) Effect of butyl lactate methacrylate content on the properties of acrylic acid copolymers. Polym Sci A 58:368–378.
Bensabeh N, Moreno A, Roig A, Monaghan OR, Ronda JC, Cádiz V, Galià M, Howdle SM, Lligadas G, Percec V (2019) Polyacrylates derived from biobased ethyl lactate solvent via SET-LRP. Biomacromol 20:2135–2147.
Bensabeh N, Moreno A, Roig A, Rahimzadeh M, Rahimi K, Ronda JC, Cádiz V, Galià M, Percec V, Rodriguez-Emmenegger C, Lligadas G (2020) Photoinduced upgrading of lactic acid-based solvents to block copolymer surfactants. ACS Sustain Chem Eng 8:1276–1284.
Lomège J, Lapinte V, Negrell C, Robin JJ, Caillol S (2019) Fatty acid-based radically polymerizable monomers: from novel poly(meth)acrylates to cutting-edge properties. Biomacromol 20:4–26.
Strehmel V, Strunk D, Heinz M, Walther S (2020) A green step to new monomers and their polymerization. Chem Select 5:12109–12114.
Ding R, Du Y, Goncalves R, Francis L, Reineke TM (2019) Sustainable near UV-curable acrylates based on natural phenolics for stereolithography 3D printing. Polym Chem 10:1067–1077.
Al-Odayni AB, Saeed WS, Yacine A, Ahmed BA, Alrahlah A, Al-Kahtani A, Aouak T (2020) New monomer based on eugenol methacrylate, synthesis, polymerization and copolymerization with methyl methacrylate–characterization and thermal properties. Polymers 12:160.
Atkinson RL, Monaghan OR, Elsmore MT, Topham PD, Toolan DTW, Derry MJ, Taresco V, Stockman RA, De Focatiis DSA, Irvine DJ, Howdle SM (2021) RAFT polymerisation of renewable terpene (meth)acrylates and the convergent synthesis of methacrylate–acrylate–methacrylate triblock copolymers. Polym Chem 12:3177–3189.
Ul M, Taresco V, Liguori A, Gualandi C, Howdle SM (2020) Synthesis of novel carvone (meth)acrylate monomers for the production of hydrophilic polymers with high terpene content. Polym Int 70:499–505.
Worzakowska M (2018) High chemical and solvent resistant, branched terpene methacrylate polymers-Preparation, thermal properties, and decomposition mechanism. Polym Adv Technol 29:1414–1425.
Sainz MF, Souto JA, Regentova D, Johansson MKG, Timhagen ST, Irvine DJ, Buijsen P, Koning CE, Stockman RA, Howdle SM (2016) A facile and green route to terpene derived acrylate and methacrylate monomers and simple free radical polymerization to yield new renewable polymers and coatings. Polym Chem 7:2882–2887.
Zweifel G, Brown HC (1964) Hydroboration of terpenes. II. The hydroboration of α-and β-pinene-the absolute configuration of the dialkylborane from the hydroboration of α-pinene. J Am Chem Soc 86:393–397.
Cravero RM, González-Sierra M, Labadie GR (2003) Convergent approaches to saudin intermediates. Helv Chim Acta 86:2741–2753.
Elamparuthi E, Fellay C, Neuburger M, Gademann K (2012) Total synthesis of cyrneine A. Angew Chemie-Int Ed 51:4071–4073.
Rai P, Maji K, Maji B (2019) Photoredox/cobalt dual catalysis for visible-light-mediated alkene–alkyne coupling. Org Lett 21:3755–3759.
Lima MS, Costa CSMF, Coelho JFJ, Fonseca AC, Serra AC (2018) A simple strategy toward the substitution of styrene by sobrerol-based monomers in unsaturated polyester resins. Green Chem 20:4880–4890.
Worzakowska M (2018) Chemical modification of potato starch by graft copolymerization with citronellyl methacrylate. J Polym Environm 26:1613–1624.
Worzakowska M (2020) The preparation, physicochemical and thermal properties of the high moisture, solvent and chemical resistant starch-g-poly(geranyl methacrylate) copolymers. J Therm Anal Calorim 140:189–198.
Worzakowska M (2019) Novel starch-g-copolymers obtained using acrylate monomers prepared from two geometric isomers of terpene alcohol. Eur Polym J 110:265–275.
Podgórski M (2010) Synthesis and characterization of novel dimethacrylates of different chain lengths as possible dental resins. Dental Mater 26:e188–e194.
Podgórski M (2011) Synthesis and characterization of acetyloxypropylene dimethacrylate as a dental monomer. Dental Mater 27:748–754.
Tuncel K, Ecevit K, Kenesci K, Piskin E (1996) Nonswellable and swellable ethylene glycol dimethacrylate-acrylic acid copolymer microspheres. J Polym Sci A Polym Chem 34:45–55.
Chen J, Garcia ES, Zimmerman SC (2020) Intramolecularly cross-linked polymers: from structure to function with applications as artificial antibodies and artificial enzymes. Acc Chem Res 53:1244–1256.
Abedin MJ, Liepold L, Suci P, Young M, Douglas T (2009) Synthesis of a crosslinked branched polymer network in the interior of a protein cage. J Am Chem Soc 131:4346–4354.
NIST Chemistry Webbook, NIST Standard Reference Data, (2011) http://webbook.nist.gov.
Parparita E, Nistor MT, Popescu MC, Vasile C (2014) TG/FTIR/MS study on thermal decomposition of polypropylene/biomass composites. Polym Degrad Stabil 109:13–20.
Huo J, Zhao S, Pan J, Pu W, Varfolomeev MA, Emalianov DA (2022) Evolution of mass losses and evolved gases of crude oil and its SARA components during low-temperature oxidation by isothermal TG–FTIR analyses. J Therm Anal Calorim 147:4099–4112.
Sharma A, Mohanty B (2021) Non-isothermal TG/DTG-FTIR kinetic study for devolatilization of Dalbergia sissoo wood under nitrogen atmosphere. J Therm Anal Calorim 146:865–879.
Qiu T, Ge F, Li C, Lu S (2021) Study of the thermal degradation of flame-retardant polyester GFRP using TGA and TG-FTIR-GC/MS. J Therm Anal Calorim. https://doi.org/10.1007/s10973-021-10895-z.
The NIST mass spectral search program for the NIST/EPA/NIH mass spectral library, version 2.0f, build Jan 25 (2008) software by S Stein, Y Mirokhin, D Tchekhovskoi, G Mallard, data evolution by A Mikaia, V Zaikin, J Little, D Zhu, W White, D Sparkman.
Li B, Liu G, Sun W, Ye L, Bi M, Gao W (2021) Experimental and theoretical study on kinetic behaviour of coal gangue and raw coal using model reconstruction. J Therm Anal Calorim 144:463–477.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Gregory Rutledge.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Worzakowska, M. TG/DSC/FTIR/QMS analysis of environmentally friendly poly(citronellyl methacrylate)-co-poly(benzyl methacrylate) copolymers. J Mater Sci 58, 2005–2024 (2023). https://doi.org/10.1007/s10853-022-08089-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-022-08089-5