Abstract
Novel red-emitting B, N, S-co-doped carbon dots (B,N,S-CDs) were prepared via a one-step hydrothermal carbonization. The obtained B,N,S-CDs are found to be responsive to the pH changes, i.e., the fluorescence intensities obviously change with the watershed of pH 7.4, accompanied by a blue-shift of the fluorescence emission wavelengths with the pH from 1 to 13. The surface state and size of the B, N, S-CDs are responsible for the fantastic responses. Furthermore, introduction of Ag+ into the B, N, S-CDs resulted in fluorescence quenching and apparent changes of extinction value, thereby the quantitative assay can be successfully realized where a linear range of 0.1–400 and 0–700 μM with a detection limit of 38 and 107 nM is obtained, respectively. The mechanism might be due to static quenching and induced electron/energy transfer (ET) resulted from the formation of non-fluorescent complex. Strikingly, Ag+ can be visualized via evident color changes from red to navy blue under UV light and from maroon to dark brown under sunlight, respectively. These findings indicate that the as-obtained B, N, S-CDs possess a great potential as a dual probe of pH and Ag+ ions for biosensing.
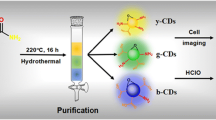
Graphical Abstract
The red-emitting fluorescent B, N, S-CDs with a fantastic pH-sensitive feature, as a dual-mode nanosensor via colorimetric and fluorescence outputs, were applied to detect Ag+ ions with the LOD of 107 and 38 nM, respectively.








Similar content being viewed by others
References
Shan L, Li Z, Xueyu F, Xie Z, Zheng M (2021) Carbon dots-based fluorescence and UV–vis absorption dual-modal sensors for Ag+ and l-cysteine detection. Dyes Pigments 187:109126. https://doi.org/10.1016/j.dyepig.2020.109126
Feng J, Chen S, Yu YL, Wang JH (2020) Red-emission hydrophobic porphyrin structure carbon dots linked with transferrin for cell imaging. Talanta 217:121014. https://doi.org/10.1016/j.talanta.2020.121014
Zhang Y, Zhou K, Qiu Y, Xia L, Xia Z, Zhang K, Qifeng F (2021) Strongly emissive formamide-derived N-doped carbon dots embedded Eu(III)-based metal-organic frameworks as a ratiometric fluorescent probe for ultrasensitive and visual quantitative detection of Ag+. Sens Actua B: Chem 339:129922. https://doi.org/10.1016/j.snb.2021.129922
Wang F, Lu Y, Chen Y, Sun J, Liu Y (2018) Colorimetric nanosensor based on the aggregation of AuNP triggered by carbon quantum dots for detection of Ag+ Ions. ACS Sustain Chem Eng 6:3706–3713. https://doi.org/10.1021/acssuschemeng.7b04067
Zhao J-L, Luo Q-Y, Ruan Q et al (2021) Red/Green tunable-emission carbon nanodots for smart visual precision pH sensing. Chem Mater 33:6091–6098. https://doi.org/10.1021/acs.chemmater.1c01620
Pal A, Ahmad K, Dutta D, Chattopadhyay A (2019) Boron doped carbon dots with unusually high photoluminescence quantum yield for ratiometric intracellular pH sensing. Chemphyschem 20:1018–1027. https://doi.org/10.1002/cphc.201900140
Xu S, He X, Huang Y et al (2020) Lysosome-targeted ratiometric fluorescent sensor for monitoring pH in living cells based on one-pot-synthesized carbon dots. Mikrochim Acta 187:478. https://doi.org/10.1007/s00604-020-04462-w
Tian Y, Chen Y, Chen M, Song ZL, Xiong B, Zhang XB (2021) Peroxidase-like Au@Pt nanozyme as an integrated nanosensor for Ag(+) detection by LSPR spectroscopy. Talanta 221:121627. https://doi.org/10.1016/j.talanta.2020.121627
Hosoba M, Oshita K, Katarina RK, Takayanagi T, Oshima M, Motomizu S (2009) Synthesis of novel chitosan resin possessing histidine moiety and its application to the determination of trace silver by ICP-AES coupled with triplet automated-pretreatment system. Anal Chim Acta 639:51–56. https://doi.org/10.1016/j.aca.2009.02.050
Ghoochani Moghadam A, Rajabi M, Hemmati M, Asghari A (2017) Development of effervescence-assisted liquid phase microextraction based on fatty acid for determination of silver and cobalt ions using micro-sampling flame atomic absorption spectrometry. J Molecular Liquids 242:1176–1183. https://doi.org/10.1016/j.molliq.2017.07.038
Kim JH, Kim KB, Park JS, Min N (2017) Single cytosine-based electrochemical biosensor for low-cost detection of silver ions. Sens Actuators B: Chem 245:741–746. https://doi.org/10.1016/j.snb.2017.01.181
Zhao D, Huang Y, Ouyang H et al (2022) Facile preparation of Cu-doped carbon dots for naked-eye discrimination of phenylenediamine isomers and highly sensitive ratiometric fluorescent detection of H2O2. Talanta 239:123110. https://doi.org/10.1016/j.talanta.2021.123110
Yang Y, Wang C, Shu Q et al (2022) Facile one-step fabrication of Cu-doped carbon dots as a dual-selective biosensor for detection of pyrophosphate ions and measurement of pH. Spectrochim Acta A Mol Biomol Spectrosc 268:120681. https://doi.org/10.1016/j.saa.2021.120681
Hu Y, Ji W, Qiao J, Li H, Zhang Y, Luo J (2021) Simple and Sensitive Multi-components detection using synthetic nitrogen-doped carbon dots based on soluble starch. J Fluoresc 31:1379–1392. https://doi.org/10.1007/s10895-021-02764-7
Zhu Z, Zhai Y, Li Z et al (2019) Red carbon dots: optical property regulations and applications. Materials Today 30:52–79. https://doi.org/10.1016/j.mattod.2019.05.003
Zhao N, Wang Y, Hou S, Zhao L (2020) Functionalized carbon quantum dots as fluorescent nanoprobe for determination of tetracyclines and cell imaging. Mikrochim Acta 187:351. https://doi.org/10.1007/s00604-020-04328-1
Yu J, Yong X, Tang Z, Yang B, Lu S (2021) Theoretical understanding of structure-property relationships in luminescence of carbon dots. J Phys Chem Lett 12:7671–7687. https://doi.org/10.1021/acs.jpclett.1c01856
Cui H, Yang J, Lu H, Li L, Zhu X, Ding Y (2022) Near-infrared carbon dots for cell imaging and detecting ciprofloxacin by label-free fluorescence sensor based on aptamer. Mikrochim Acta 189:170. https://doi.org/10.1007/s00604-022-05273-x
Zhang M, Su R, Zhong J et al (2019) Red/orange dual-emissive carbon dots for pH sensing and cell imaging. Nano Research 12:815–821. https://doi.org/10.1007/s12274-019-2293-z
Huang S, Yang E, Yao J, Liu Y, Xiao Q (2018) Red emission nitrogen, boron, sulfur co-doped carbon dots for “on-off-on” fluorescent mode detection of Ag(+) ions and l-cysteine in complex biological fluids and living cells. Anal Chim Acta 1035:192–202. https://doi.org/10.1016/j.aca.2018.06.051
Peng B, Fan M, Xu J et al (2020) Dual-emission ratio fluorescent probes based on carbon dots and gold nanoclusters for visual and fluorescent detection of copper ions. Mikrochim Acta 187:660. https://doi.org/10.1007/s00604-020-04641-9
Li C, Zhao J, Chen Y et al (2018) A carbon dots/rutin system for colorimetric and fluorimetric dual mode detection of Al(3+) in aqueous solution. Analyst 143:5467–5473. https://doi.org/10.1039/c8an00962g
Liu Y, Duan W, Song W et al (2017) Red emission B, N, S-co-doped carbon dots for colorimetric and fluorescent dual mode detection of Fe(3+) Ions in complex biological fluids and living cells. ACS Appl Mater Interfaces 9:12663–12672. https://doi.org/10.1021/acsami.6b15746
Li T, Shuang E, Wang J, Chen X (2019) Regulating the properties of carbon dots via a solvent-involved molecule fusion strategy for improved sensing selectivity. Anal Chim Acta 1088:107–115. https://doi.org/10.1016/j.aca.2019.08.027
Li T, Shi W, Shuang E, Mao Q, Chen X (2021) Green preparation of carbon dots with different surface states simultaneously at room temperature and their sensing applications. J Colloid Interface Sci 591:334–342. https://doi.org/10.1016/j.jcis.2021.02.024
Song Z, Quan F, Xu Y, Liu M, Cui L, Liu J (2016) Multifunctional N, S co-doped carbon quantum dots with pH- and thermo-dependent switchable fluorescent properties and highly selective detection of glutathione. Carbon 104:169–178. https://doi.org/10.1016/j.carbon.2016.04.003
Ng HKM, Lim GK, Leo CP (2021) Comparison between hydrothermal and microwave-assisted synthesis of carbon dots from biowaste and chemical for heavy metal detection: a review. Microchem J 165:106116. https://doi.org/10.1016/j.microc.2021.106116
Pandey SC, Kumar A, Sahu SK (2020) Single step green synthesis of carbon dots from Murraya koenigii leaves; a unique turn-off fluorescent contrivance for Selective Sensing of Cd (II) ion. J Photochem Photobiol A: Chem 400:112620. https://doi.org/10.1016/j.jphotochem.2020.112620
Lu W, Gong X, Nan M, Liu Y, Shuang S, Dong C (2015) Comparative study for N and S doped carbon dots: synthesis, characterization and applications for Fe(3+) probe and cellular imaging. Anal Chim Acta 898:116–127. https://doi.org/10.1016/j.aca.2015.09.050
Xu Q, Su R, Zhong J et al (2018) Synthesis of highly fluorescent yellow-green N-doped carbon nanorings for pH variation detection and bioimaging. Particle Particle Syst Charact 35:1800276. https://doi.org/10.1002/ppsc.201800276
Peng B, Xu J, Fan M et al (2020) Smartphone colorimetric determination of hydrogen peroxide in real samples based on B N, and S co-doped carbon dots probe. Anal Bioanal Chem 412:861–870. https://doi.org/10.1007/s00216-019-02284-1
Zhong D, Miao H, Yang K, Yang X (2016) Carbon dots originated from carnation for fluorescent and colorimetric pH sensing. Materials Letters 166:89–92. https://doi.org/10.1016/j.matlet.2015.12.061
Luo L, Wang P, Wang Y, Wang F (2018) pH assisted selective detection of Hg(II) and Ag(I) based on nitrogen-rich carbon dots. Sens Actua B: Chem 273:1640–1647. https://doi.org/10.1016/j.snb.2018.07.090
Ding H, Wei JS, Xiong HM (2014) Nitrogen and sulfur co-doped carbon dots with strong blue luminescence. Nanoscale 6:13817–13823. https://doi.org/10.1039/c4nr04267k
Jin X, Sun X, Chen G et al (2015) pH-sensitive carbon dots for the visualization of regulation of intracellular pH inside living pathogenic fungal cells. Carbon 81:388–395. https://doi.org/10.1016/j.carbon.2014.09.071
Dang DK, Sundaram C, Ngo Y-LT, Chung JS, Kim EJ, Hur SH (2018) One pot solid-state synthesis of highly fluorescent N and S co-doped carbon dots and its use as fluorescent probe for Ag+ detection in aqueous solution. Sens Actuat B: Chem 255:3284–3291. https://doi.org/10.1016/j.snb.2017.09.155
Hola K, Bourlinos AB, Kozak O et al (2014) Photoluminescence effects of graphitic core size and surface functional groups in carbon dots: COO− induced red-shift emission. Carbon 70:279–286. https://doi.org/10.1016/j.carbon.2014.01.008
Long Y-M, Zhou C-H, Zhang Z-L et al (2012) Shifting and non-shifting fluorescence emitted by carbon nanodots. J Mater Chem 22:5917–5920. https://doi.org/10.1039/C2JM30639E
Sykora M, Mangolini L, Schaller RD, Kortshagen U, Jurbergs D, Klimov VI (2008) Size-dependent intrinsic radiative decay rates of silicon nanocrystals at large confinement energies. Phys Rev Lett 100:067401. https://doi.org/10.1103/PhysRevLett.100.067401
Xu Z-Q, Lan J-Y, Jin J-C et al (2015) Mechanistic studies on the reversible photophysical properties of carbon nanodots at different pH. Colloid Surfac B: Biointerfaces 130:207–214. https://doi.org/10.1016/j.colsurfb.2015.04.012
Fiuza T, Gomide G, Campos AFC, Messina F, Depeyrot J (2019) On the colloidal stability of nitrogen-rich carbon nanodots aqueous dispersions. C-J Carbon Res 5:74. https://doi.org/10.3390/c5040074
Wang X, Qu K, Xu B, Ren J, Qu X (2011) Microwave assisted one-step green synthesis of cell-permeable multicolor photoluminescent carbon dots without surface passivation reagents. J Mater Chem 21:2445–2450. https://doi.org/10.1039/C0JM02963G
Ding H, Wei JS, Zhang P, Zhou ZY, Gao QY, Xiong HM (2018) Solvent-controlled synthesis of highly luminescent carbon dots with a wide color gamut and narrowed emission peak widths. Small 14:e1800612. https://doi.org/10.1002/smll.201800612
Feng J, Zhao X, Bian W, Tang X (2019) Microwave-assisted synthesis of nitrogen-rich carbon dots as effective fluorescent probes for sensitive detection of Ag+. Mater Chem Front 3:2751–2758. https://doi.org/10.1039/c9qm00624a
Lu H, Li C, Wang H, Wang X, Xu S (2019) Biomass-derived Sulfur, nitrogen co-doped carbon dots for colorimetric and fluorescent dual mode detection of Silver (I) and Cell imaging. ACS Omega 4:21500–21508. https://doi.org/10.1021/acsomega.9b03198
Chen X, Bai J, Yuan G, Zhang L, Ren L (2021) One-pot preparation of nitrogen-doped carbon dots for sensitive and selective detection of Ag+ and glutathione. Microchemical Journal 165:106156. https://doi.org/10.1016/j.microc.2021.106156
Li Q, Bai Z, Xi X et al (2021) Rapid microwave-assisted green synthesis of guanine-derived carbon dots for highly selective detection of Ag+ in aqueous solution. Spectrochim Acta Part A: Molecul Biomol Spectrosc 248:119208. https://doi.org/10.1016/j.saa.2020.119208
Gao X, Lu Y, Zhang R et al (2015) One-pot synthesis of carbon nanodots for fluorescence turn-on detection of Ag+ based on the Ag+-induced enhancement of fluorescence. J Mater Chem C 3:2302–2309. https://doi.org/10.1039/c4tc02582b
Shen C, Ge S, Pang Y et al (2017) Facile and scalable preparation of highly luminescent N S co-doped graphene quantum dots and their application for parallel detection of multiple metal ions. J Mater Chem B 5:6593–6600. https://doi.org/10.1039/c7tb00506g
Pan X, Zhang Y, Sun X et al (2018) Carbon dots originated from methyl red with molecular state and surface state controlled emissions for sensing and imaging. J Luminescence 204:303–311. https://doi.org/10.1016/j.jlumin.2018.08.007
Jiang K, Sun S, Zhang L, Wang Y, Cai C, Lin H (2015) Bright-yellow-emissive N-doped carbon dots: preparation, cellular imaging, and bifunctional sensing. ACS Appl Mater Interfaces 7:23231–23238. https://doi.org/10.1021/acsami.5b07255
Junaid HM, Solangi AR, Batool M (2021) Carbon dots as naked eye sensors. Analyst 146:2463–2474. https://doi.org/10.1039/d0an02399j
Shi L, Hou Z, Zhang C et al (2019) Concentration-dependent multicolor fluorescent carbon dots for colorimetric and fluorescent bimodal detections of Fe3+ and l-ascorbic acid. Analytical Methods 11:669–676. https://doi.org/10.1039/c8ay02486c
Chang D, Shi L, Zhang Y et al (2020) Smilax China-derived yellow-fluorescent carbon dots for temperature sensing, Cu(2+) detection and cell imaging. Analyst 145:2176–2183. https://doi.org/10.1039/d0an00102c
Atchudan R, Edison TNJI, Perumal S, Muthuchamy N, Lee YR (2020) Hydrophilic nitrogen-doped carbon dots from biowaste using dwarf banana peel for environmental and biological applications. Fuel 275:117821. https://doi.org/10.1016/j.fuel.2020.117821
Zulfajri M, Gedda G, Chang CJ, Chang YP, Huang GG (2019) Cranberry beans derived carbon dots as a potential fluorescence sensor for selective detection of Fe(3+) Ions in aqueous solution. ACS Omega 4:15382–15392. https://doi.org/10.1021/acsomega.9b01333
Acknowledgements
The authors would like to acknowledge financial support from the National Natural Science Foundation of China (Grant No. 21705003).
Author information
Authors and Affiliations
Contributions
NX did data curation, writing—original draft. CW and QS investigated the study. QX and PZ performed formal analysis. SZ done investigation, methodology, and supervision. CZ gave resources. JD was involved in investigation, methodology, project administration, resources, supervision, writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Additional information
Handling Editor: Andrea de Camargo.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Du, J., Xu, N., Wang, C. et al. Red emission B, N, S-co-doped carbon dots with pH-responsive functionality for colorimetric and fluorescent dual-mode detection of Ag+ ions. J Mater Sci 57, 21693–21708 (2022). https://doi.org/10.1007/s10853-022-07988-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-022-07988-x