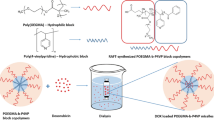
Abstract
In this study, a thermo-responsive poly(N-isopropylacrylamide-b-lauryl acrylate) (PNIPAAm-b-PLA) as a smart amphiphilic block copolymer was fabricated with tailored molecular weight through a controlled polymerization method, i.e., reversible addition-fragmentation chain-transfer (RAFT). Initially, the homopolymer of NIPAAm was synthesized and applied in the role of macro-RAFT agent to copolymerize with LA monomer. The PNIPAAm-b-PLA nanosystem was self-assembled and organized stable nanomicelles with a low amount of critical micelle concentration (CMC) of 2.07 mg L−1 and small spherical dimensions. The anti-cancer therapeutic cargo, docetaxel (DTX) was encapsulated in a hydrophobic interior region of block copolymer (micelle core) via Van der Waals interactions with high loading efficiency. In vitro drug liberation profile from polymeric micelles demonstrated that DTX delivery was thermo-sensitive with a sustained drug release rate. The safety and anti-cancer effects of DTX and as-prepared micellar structures were investigated through an MTT assay on MCF-7 cells. The results exhibited that DTX loaded polymeric micelles revealed a comparable amount of toxicity to free drugs. It was concluded that this system emerges as a potentially favorable and powerful intracellular delivery of antitumor drug system in chemotherapy.
Graphical abstract
Thermo-responsive PNIPAAm-b-PLA was fabricated through RAFT controlled method. The DTX was encapsulated in the self-assembled nanomicelles with high loading efficiency. In vitro drug liberation profile from polymeric micelles demonstrated that DTX delivery was thermo-sensitive with a sustained drug release rate. The results revealed that this system could be favorable delivery system to treat breast cancer.












Similar content being viewed by others
References
Farjadian F, Ghasemi A, Gohari O, Roointan A, Karimi M, Hamblin MR (2019) Nanopharmaceuticals and nanomedicines currently on the market: challenges and opportunities. Nanomedicine 14(1):93–126. https://doi.org/10.2217/nnm-2018-0120
Geraili A, Xing M, Mequanint K (2021) Design and fabrication of drug-delivery systems toward adjustable release profiles for personalized treatment. View 2(5):20200126. https://doi.org/10.1002/VIW.20200126
Khan AU, Khan M, Cho MH, Khan MM (2020) Selected nanotechnologies and nanostructures for drug delivery, nanomedicine and cure. Bioprocess Biosyst Eng 43(8):1339–1357. https://doi.org/10.1007/s00449-020-02330-8
Nezhadi S, Saadat E, Handali S, Dorkoosh F (2021) Nanomedicine and chemotherapeutics drug delivery: challenges and opportunities. J Drug Target 29(2):185–198. https://doi.org/10.1080/1061186X.2020.1808000
van der Koog L, Gandek TB, Nagelkerke A (2021) Liposomes and extracellular vesicles as drug delivery systems: a comparison of composition, pharmacokinetics, and functionalization. Adv Healthcare Mater 11(5):2100639. https://doi.org/10.1002/adhm.202100639
Begines B, Ortiz T, Pérez-Aranda M, Martínez G, Merinero M, Argüelles-Arias F et al (2020) Polymeric nanoparticles for drug delivery: recent dDevelopments and future prospects. Nanomaterials (Basel) 10(7):1403. https://doi.org/10.3390/nano10071403
Hoseini-Ghahfarokhi M, Mirkiani S, Mozaffari N, Sadatlu MAA, Ghasemi A, Abbaspour S et al (2020) Applications of graphene and graphene oxide in smart drug/dene delivery: is the world still flat? Int J Nanomed 15:9469. https://doi.org/10.2147/IJN.S265876
Singh S, Marwah H, Rawat P, Awasthi S, Singh L, Rajak H et al (2022) Chapter 4–Hybrid Nanogel Systems For Drug Delivery. In: Kesharwani P, Jain NK (eds) Hybrid Nanomaterials for Drug Delivery. Woodhead Publishing, pp 85–100. https://doi.org/10.1016/B978-0-323-85754-3.00008-3
Farjadian F, Roointan A, Mohammadi-Samani S, Hosseini M (2019) Mesoporous silica nanoparticles: synthesis, pharmaceutical applications, biodistribution, and biosafety assessment. Chem Eng J 359:684–705. https://doi.org/10.1016/j.cej.2018.11.156
Akbarian M, Gholinejad M, Mohammadi-Samani S, Farjadian F (2022) Theranostic mesoporous silica nanoparticles made of multi-nuclear gold or carbon quantum dots particles serving as pH responsive drug delivery system. Microporous Mesoporous Mater 329:111512. https://doi.org/10.1016/j.micromeso.2021.111512
Mi P (2020) Stimuli-responsive nanocarriers for drug delivery, tumor imaging, therapy and theranostics. Theranostics 10(10):4557. https://doi.org/10.7150/thno.38069
Lombardo D, Kiselev MA, Caccamo MT (2019) Smart nanoparticles for drug delivery application: development of versatile nanocarrier platforms in biotechnology and nanomedicine. J Nanomater 2019:1–26. https://doi.org/10.1155/2019/3702518
Imran M, Shah MR (2018) Amphiphilic block copolymers–based micelles for drug delivery. Design and Development of New Nanocarriers. Elsevier, London, pp 365–400. https://doi.org/10.1016/B978-0-12-813627-0.00010-7
Bodratti AM, Alexandridis P (2018) Amphiphilic block copolymers in drug delivery: advances in formulation structure and performance. Expert Opin Drug Deliv 15(11):1085–1104. https://doi.org/10.1080/17425247.2018.1529756
Deshmukh AS, Chauhan PN, Noolvi MN, Chaturvedi K, Ganguly K, Shukla SS et al (2017) Polymeric micelles: basic research to clinical practice. Int J Pharm 532(1):249–268. https://doi.org/10.1016/j.ijpharm.2017.09.005
Zhou Q, Zhang L, Yang T, Wu H (2018) Stimuli-responsive polymeric micelles for drug delivery and cancer therapy. Int J Nanomed 13:2921. https://doi.org/10.2147/IJN.S158696
Aliabadi HM, Lavasanifar A (2006) Polymeric micelles for drug delivery. Expert Opin Drug Deliv 3(1):139–162. https://doi.org/10.1517/17425247.3.1.139
Bajpai AK, Shukla SK, Bhanu S, Kankane S (2008) Responsive polymers in controlled drug delivery. Prog Polym Sci 33(11):1088–1118. https://doi.org/10.1016/j.progpolymsci.2008.07.005
Hosseini M, Farjadian F, Makhlouf ASH (2016) Smart stimuli-responsive nano-sized hosts for drug delivery Industrial Applications for Intelligent Polymers and Coatings. Springer, Cham. https://doi.org/10.1007/978-3-319-26893-4_1
Cao M, Wang Y, Hu X, Gong H, Li R, Cox H et al (2019) Reversible thermoresponsive peptide–PNIPAM hydrogels for controlled drug delivery. Biomacromol 20(9):3601–3610. https://doi.org/10.1021/acs.biomac.9b01009
Huang N, Wang J, Cheng X, Xu Y, Li W (2020) Fabrication of PNIPAM-chitosan/decatungstoeuropate/silica nanocomposite for thermo/pH dual-stimuli-responsive and luminescent drug delivery system. J Inorg Biochem 211:111216. https://doi.org/10.1016/j.jinorgbio.2020.111216
Skandalis A, Selianitis D, Pispas S (2021) PnBA-b-PNIPAM-b-PDMAEA thermo-responsive triblock terpolymers and their quaternized analogs as gene and drug delivery vectors. Polymers 13(14):2361. https://doi.org/10.3390/polym13142361
Massoumi B, Ghamkhari A, Agbolaghi S (2018) Dual stimuli-responsive poly(succinyloxyethylmethacrylate-b-N-isopropylacrylamide) block copolymers as nanocarriers and respective application in doxorubicin delivery. Int J Polym Mater Polym Biomater 67(2):101–109. https://doi.org/10.1080/00914037.2017.1300901
Pourjavadi A, Tehrani ZM (2017) Poly(N-isopropylacrylamide)-coated β-cyclodextrin–capped magnetic mesoporous silica nanoparticles exhibiting thermal and pH dual response for triggered anticancer drug delivery. Int J Polym Mater Polym Biomater 66(7):336–348. https://doi.org/10.1080/00914037.2016.1217531
Li P, Xu R, Wang W, Li X, Xu Z, Yeung KW et al (2013) Thermosensitive poly(N-isopropylacrylamide-co-glycidyl methacrylate) microgels for controlled drug release. Colloids Surf B 101:251–255. https://doi.org/10.1016/j.colsurfb.2012.07.009
Maheswari B, Jagadeesh Babu P, Agarwal M (2014) Role of N-vinyl-2-pyrrolidinone on the thermoresponsive behavior of PNIPAm hydrogel and its release kinetics using dye and vitamin-B12 as model drug. J Biomater Sci Polym Ed 25(3):269–286. https://doi.org/10.1080/09205063.2013.854149
Conzatti G, Cavalie S, Combes C, Torrisani J, Carrere N, Tourrette A (2017) PNIPAM grafted surfaces through ATRP and RAFT polymerization: chemistry and bioadhesion. Colloids Surf B 151:143–155. https://doi.org/10.1016/j.colsurfb.2016.12.007
Akimoto J, Nakayama M, Okano T (2014) Temperature-responsive polymeric micelles for optimizing drug targeting to solid tumors. J Control Release 193:2–8. https://doi.org/10.1016/j.jconrel.2014.06.062
Nakayama M, Okano T, Miyazaki T, Kohori F, Sakai K, Yokoyama M (2006) Molecular design of biodegradable polymeric micelles for temperature-responsive drug release. J Control Release 115(1):46–56. https://doi.org/10.1016/j.jconrel.2006.07.007
Yang M, Ding Y, Zhang L, Qian X, Jiang X, Liu B (2007) Novel thermosensitive polymeric micelles for docetaxel delivery. J Biomed Mater Res Part A 81(4):847–857. https://doi.org/10.1002/jbm.a.31129
Cheng C, Wei H, Zhang XZ, Cheng SX, Zhuo RX (2009) Thermo-triggered and biotinylated biotin-P (NIPAAm-co-HMAAm)-b-PMMA micelles for controlled drug release. J Biomed Mater Res Part A 88(3):814–822. https://doi.org/10.1002/jbm.a.31770
Peng C-L, Tsai H-M, Yang S-J, Luo T-Y, Lin C-F, Lin W-J et al (2011) Development of thermosensitive poly(n-isopropylacrylamide-co-((2-dimethylamino) ethyl methacrylate))-based nanoparticles for controlled drug release. Nanotechnology 22(26):265608. https://doi.org/10.1088/0957-4484/22/26/265608
Rezaei SJT, Nabid MR, Niknejad H, Entezami AA (2012) Folate-decorated thermoresponsive micelles based on star-shaped amphiphilic block copolymers for efficient intracellular release of anticancer drugs. Int J Pharm 437(1–2):70–79. https://doi.org/10.1016/j.ijpharm.2012.07.069
Panja S, Dey G, Bharti R, Kumari K, Maiti T, Mandal M et al (2016) Tailor-made temperature-sensitive micelle for targeted and on-demand release of anticancer drugs. ACS Appl Mater Interfaces 8(19):12063–12074. https://doi.org/10.1021/acsami.6b03820
Qin S, Geng Y, Discher DE, Yang S (2006) Temperature-controlled assembly and release from polymer vesicles of poly(ethylene oxide)-block-poly(N-isopropylacrylamide). Adv Mater 18(21):2905–2909. https://doi.org/10.1002/adma.200601019
Sun X-L, Tsai P-C, Bhat R, Bonder E, Michniak-Kohn B, Pietrangelo A (2015) Thermoresponsive block copolymer micelles with tunable pyrrolidone-based polymer cores: structure/property correlations and application as drug carriers. J Mater Chem B 3(5):814–823. https://doi.org/10.1039/C4TB01494D
Chung J, Yokoyama M, Yamato M, Aoyagi T, Sakurai Y, Okano T (1999) Thermo-responsive drug delivery from polymeric micelles constructed using block copolymers of poly(N-isopropylacrylamide) and poly(butylmethacrylate). J Control Release 62(1–2):115–127. https://doi.org/10.1016/S0168-3659(99)00029-2
Zhang L, Guo R, Yang M, Jiang X, Liu B (2007) Thermo and pH dual-responsive nanoparticles for anti-cancer drug delivery. Adv Mater 19(19):2988–2992. https://doi.org/10.1002/adma.200601817
Wang X, Li S, Wan Z, Quan Z, Tan Q (2014) Investigation of thermo-sensitive amphiphilic micelles as drug carriers for chemotherapy in cholangiocarcinoma in vitro and in vivo. Int J Pharm 463(1):81–88. https://doi.org/10.1016/j.ijpharm.2013.12.046
Ghasemi S, Karim S (2017) Mizoroki-Heck cross-coupling reaction of haloarenes mediated by a well-controlled modified polyacrylamide brush grafted silica/pd nanoparticle system. Bull Chem Soc Jpn 90(5):485–490. https://doi.org/10.1246/bcsj.20160374
Entezar-Almahdi E, Heidari R, Ghasemi S, Mohammadi-Samani S, Farjadian F (2021) Integrin receptor mediated pH-responsive nano-hydrogel based on histidine-modified poly(aminoethyl methacrylamide) as targeted cisplatin delivery system. J Drug Deliv Sci Technol 62:102402. https://doi.org/10.1016/j.jddst.2021.102402
Farjadian F, Ghasemi S, Andami Z, Tamami B (2020) Thermo-responsive nanocarrier based on poly(N-isopropylacrylamide) serving as a smart doxorubicin delivery system. Iran Polym J 29(3):197–207. https://doi.org/10.1007/s13726-020-00785-w
Farjadian F, Rezaeifard S, Naeimi M, Ghasemi S, Mohammadi-Samani S, Welland ME et al (2019) Temperature and pH-responsive nano-hydrogel drug delivery system based on lysine-modified poly(vinylcaprolactam). Int J Nanomed 14:6901. https://doi.org/10.2147/IJN.S214467
Huang J, Zhang H, Yu Y, Chen Y, Wang D, Zhang G et al (2014) Biodegradable self-assembled nanoparticles of poly(d, l-lactide-co-glycolide)/hyaluronic acid block copolymers for target delivery of docetaxel to breast cancer. Biomaterials 35(1):550–566. https://doi.org/10.1016/j.biomaterials.2013.09.089
Farzanfar J, Farjadian F, Roointan A, Mohammadi-Samani S, Tayebi L (2021) Assessment of pH responsive delivery of methotrexate based on PHEMA-st-PEG-DA nanohydrogels. Macromol Res 29:54–61. https://doi.org/10.1007/s13233-021-9007-6
Zhang L, Eisenberg A (1995) Multiple morphologies of "crew-cut" aggregates of polystyrene-b-poly(acrylic acid) block copolymers. Science 268(5218):1728–1731. https://doi.org/10.1126/science.268.5218.1728
Hami Z, Amini M, Ghazi-Khansari M, Rezayat SM, Gilani K (2014) Synthesis and in vitro evaluation of a pH-sensitive PLA–PEG–folate based polymeric micelle for controlled delivery of docetaxel. Colloids Surf, B 116:309–317. https://doi.org/10.1016/j.colsurfb.2014.01.015
Acknowledgements
The authors would greatly have appreciated the partial support of this study by the Shiraz University Research Council and the support from the Research Council of Shiraz University of Medical Sciences under Grant No.97-01-106-18498/15324.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Handling Editor: Annela M. Seddon.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ghasemi, S., Ahmadi, L. & Farjadian, F. Thermo-responsive PNIPAAm-b-PLA amphiphilic block copolymer micelle as nanoplatform for docetaxel drug release. J Mater Sci 57, 17433–17447 (2022). https://doi.org/10.1007/s10853-022-07711-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-022-07711-w