Abstract
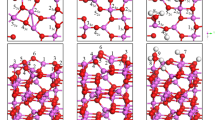
The study on the interaction of water with catalyst surface is significant for the design of efficient catalysts for the reactions with water as reactant. Herein, the DFT-D3 method was employed to explore the adsorption state and mechanism between catalyst surface and high coverage water. The results showed that single H2O molecule was more favorable dissociative adsorbed on the Co and Ni-promoted γ-Al2O3 (110) with adsorption energies of −193 and −259 kJ/mol. For the one O defective surface, the dissociated OH of H2O molecule filled into the O vacancy. The interaction mechanism between H2O molecule with the surface was the lone pair electron of adsorbed H2O overlap with the surface Lewis acid metal 3d orbit. The dissociation mechanism for single H2O molecule was dissociated H migrated to the surface metal sites located between Ow and Os. The surface phase diagram showed that Co and Ni-promoted γ-Al2O3 (110) exhibit five stable adsorption structures.
Graphical abstract









Similar content being viewed by others
References
Alberton AL, Souza MM, Schmal M (2007) Carbon formation and its influence on ethanol steam reforming over Ni/Al2O3 catalysts. Catal Today 123:257–264. https://doi.org/10.1016/j.cattod.2007.01.062
Comas J, Mariño F, Laborde M, Amadeo N (2004) Bio-ethanol steam reforming on Ni/Al2O3 catalyst. Chem Eng J 98:61–68. https://doi.org/10.1016/S1385-8947(03)00186-4
Dissanayake D, Rosynek MP, Kharas KC, Lunsford JH (1991) Partial oxidation of methane to carbon monoxide and hydrogen over a Ni/Al2O3 catalyst. J Catal 132:117–127. https://doi.org/10.1016/0021-9517(91)90252-Y
Hu D, Gao J, Ping Y et al (2012) Enhanced investigation of CO methanation over Ni/Al2O3 catalysts for synthetic natural gas production. Ind Eng Chem Res 51:4875–4886. https://doi.org/10.1021/ie300049f
Zhu X, Huo P, Zhang Y-p, Cheng D-G, Liu C-J (2008) Structure and reactivity of plasma treated Ni/Al2O3 catalyst for CO2 reforming of methane. Appl Catal, B-Environ 81:132–140. https://doi.org/10.1016/j.apcatb.2007.11.042
Yang Z, Lei Z, Ge B et al (2021) Development of catalytic combustion and CO2 capture and conversion technology. Int J Coal Sci Techn 8:377–382. https://doi.org/10.1007/S40789-021-00444-2
Ye R-P, Liao L, Reina TR et al (2021) Engineering Ni/SiO2 catalysts for enhanced CO2 methanation. Fuel 285:119151. https://doi.org/10.1016/j.fuel.2020.119151
Gao Y, Ma J, Meng F, Wang W, Li Z (2020) Solution-combusted nanosized Ni–Al2O3 catalyst for slurry CO methanation: effects of alkali/alkaline earth metal chlorides. J Mater Sci 55:16510–16521. https://doi.org/10.1007/s10853-020-05222-0
Ma W, Jacobs G, Das TK et al (2014) Fischer-Tropsch synthesis: kinetics and water effect on methane formation over 25%Co/γ-Al2O3 catalyst. Ind Eng Chem Res 53:2157–2166. https://doi.org/10.1021/ie402094b
Jacobs G, Patterson PM, Zhang Y, Das T, Li J, Davis BH (2002) Fischer-Tropsch synthesis: deactivation of noble metal-promoted Co/Al2O3 catalysts. Appl Catal, A-Gen 233:215–226. https://doi.org/10.1016/S0926-860X(02)00147-3
Jacobs G, Chaney JA, Patterson PM, Das TK, Davis BH (2004) Fischer-Tropsch synthesis: study of the promotion of Re on the reduction property of Co/Al2O3 catalysts by in situ EXAFS/XANES of Co K and Re LIII edges and XPS. Appl Catal, A-Gen 264:203–212. https://doi.org/10.1016/j.apcata.2003.12.049
Xiong H, Zhang Y, Wang S, Li J (2005) Fischer-Tropsch synthesis: the effect of Al2O3 porosity on the performance of Co/Al2O3 catalyst. Catal Commun 6:512–516. https://doi.org/10.1016/j.catcom.2005.04.018
Munnik P, Krans NA, de Jongh PE, de Jong KP (2014) Effects of drying conditions on the synthesis of Co/SiO2 and Co/Al2O3 Fischer-Tropsch catalysts. ACS Catal 4:3219–3226. https://doi.org/10.1021/cs5006772
Fatah N, Dhainaut F (2019) Analysis of particle breakage during the preparation steps of Co/Al2O3 catalysts. J Mater Sci 54:14275–14286. https://doi.org/10.1007/s10853-019-03918-6
Yang J, Dettori R, Nunes JPF et al (2021) Direct observation of ultrafast hydrogen bond strengthening in liquid water. Nature 596:531–535. https://doi.org/10.1038/s41586-021-03793-9
Wang Y-H, Zheng S, Yang W-M et al (2021) In situ Raman spectroscopy reveals the structure and dissociation of interfacial water. Nature 600:81–85. https://doi.org/10.1038/s41586-021-04068-z
Shafiee P, Alavi SM, Rezaei M, Jokar F (2022) Promoted Ni–Co–Al2O3 nanostructured catalysts for CO2 methanation. Int J Hydrogen Energ 47:2399–2411. https://doi.org/10.1016/j.ijhydene.2021.10.197
Visconti CG, Lietti L, Tronconi E, Forzatti P, Zennaro R, Finocchio E (2009) Fischer-Tropsch synthesis on a Co/Al2O3 catalyst with CO2 containing syngas. Appl Catal, A-Gen 355:61–68. https://doi.org/10.1016/j.apcata.2008.11.027
Cornaro U, Rossini S, Montanari T, Finocchio E, Busca G (2012) K-doping of Co/Al2O3 low temperature Fischer-Tropsch catalysts. Catal Today 197:101–108. https://doi.org/10.1016/j.cattod.2012.07.005
Kondo JN, Iizuka M, Domen K, Wakabayashi F (1997) IR study of H2O adsorbed on H-ZSM-5. Langmuir 13:747–750. https://doi.org/10.1021/la9607565
Garbarino G, Finocchio E, Lagazzo A et al (2014) Steam reforming of ethanol–phenol mixture on Ni/Al2O3: effect of magnesium and boron on catalytic activity in the presence and absence of sulphur. Appl Catal, B-Environ 147:813–826. https://doi.org/10.1016/j.apcatb.2013.09.030
Choong CK, Huang L, Zhong Z, Lin J, Hong L, Chen L (2011) Effect of calcium addition on catalytic ethanol steam reforming of Ni/Al2O3: II. Acidity/basicity, water adsorption and catalytic activity. Appl Catal, A-Gen 407:155–162. https://doi.org/10.1016/j.apcata.2011.08.038
Park S-J, Bae JW, Jung G-I et al (2012) Crucial factors for catalyst aggregation and deactivation on Co/Al2O3 in a slurry-phase Fischer-Tropsch synthesis. Appl Catal, A-Gen 413:310–321. https://doi.org/10.1016/j.apcata.2011.11.022
Digne M (2004) Use of DFT to achieve a rational understanding of acid-basic properties of γ-alumina surfaces. J Catal 226:54–68. https://doi.org/10.1016/j.jcat.2004.04.020
Digne M, Sautet P, Raybaud P, Euzen P, Toulhoat H (2002) Hydroxyl groups on γ-alumina surfaces: a DFT study. J Catal 211:1–5. https://doi.org/10.1006/jcat.2002.3741
Shi L, Meng S, Jungsuttiwong S et al (2020) High coverage H2O adsorption on CuAl2O4 surface: a DFT study. Appl Surf Sci 507:145162. https://doi.org/10.1016/j.apsusc.2019.145162
Xi H, Hou X, Liu Y, Qing S, Gao Z (2014) Cu-Al spinel oxide as an efficient catalyst for methanol steam reforming. Angew Chem Int Ed 53:12080–12083. https://doi.org/10.1002/anie.201405213
Shi L, Huang Y, Lu Z-H et al (2021) Surface property of the Cu doped γ-Al2O3: A density functional theory study. Appl Surf Sci 535:147651. https://doi.org/10.1016/j.apsusc.2020.147651
Feng G, Huo C-F, Li Y-W, Wang J, Jiao H (2011) Structures and energies of iron promoted γ-Al2O3 surface: a computational study. Chem Phys Lett 510:224–227. https://doi.org/10.1016/j.cplett.2011.05.035
Ma FF, Ma SH, Jiao ZY, Dai XQ (2016) Adsorption and decomposition of H2O on cobalt surfaces: a DFT study. Appl Surf Sci 384:10–17. https://doi.org/10.1016/j.apsusc.2016.05.019
Xu XL, Li JQ (2011) DFT studies on H2O adsorption and its effect on CO oxidation over spinel Co3O4 (110) surface. Surf Sci 605:1962–1967. https://doi.org/10.1016/j.susc.2011.07.013
Mohsenzadeh A, Bolton K, Richards T (2014) DFT study of the adsorption and dissociation of water on Ni (111), Ni (110) and Ni (100) surfaces. Surf Sci 627:1–10. https://doi.org/10.1016/j.susc.2014.04.006
Yu N, Zhang W-B, Wang N, Wang Y-F, Tang B-Y (2008) Water adsorption on a NiO (100) surface: a GGA+U study. J Phys Chem C 112:452–457. https://doi.org/10.1021/jp070641h
Ken O, Bin L, Jin Z, Jordan KD, Jinlong Y, Hrvoje P (2005) Wet electrons at the H2O/TiO2(110) surface. Science 308:1154–1158. https://doi.org/10.1126/science.1109366
Yu X, Zhang X, Wang H, Feng G (2017) High coverage water adsorption on the CuO(111) surface. Appl Surf Sci 425:803–810. https://doi.org/10.1016/j.apsusc.2017.07.086
Zhang J, Zhang R, Wang B, Ling L (2016) Insight into the adsorption and dissociation of water over different CuO(111) surfaces: the effect of surface structures. Appl Surf Sci 364:758–768. https://doi.org/10.1016/j.apsusc.2015.12.211
Yu X, Zhang X, Wang S, Feng G (2015) A computational study on water adsorption on Cu2O(111) surfaces: the effects of coverage and oxygen defect. Appl Surf Sci 343:33–40. https://doi.org/10.1016/j.apsusc.2015.03.065
Hass KC, Schneider WF, Curioni A, Andreoni W (1998) The chemistry of water on alumina surfaces: Reaction dynamics from first principles. Science 282:265–268. https://doi.org/10.1126/science.282.5387.265
Lejaeghere K, Bihlmayer G, Bjorkman T et al (2016) Reproducibility in density functional theory calculations of solids. Science 351:aad3000. https://doi.org/10.1126/science.aad3000
Lejaeghere K, Van Speybroeck V, Van Oost G, Cottenier S (2014) Error estimates for solid-state density-functional theory predictions: An overview by means of the ground-state elemental crystals. Crit Rev Solid State Mater Sci 39:1–24. https://doi.org/10.1080/10408436.2013.772503
Wang F, Ma J, Xin S et al (2020) Resolving the puzzle of single-atom silver dispersion on nanosized gamma-Al2O3 surface for high catalytic performance. Nat Commun 11:529. https://doi.org/10.1038/s41467-019-13937-1
Li J, Shi L, Feng G, Shi Z, Sun C, Kong D (2020) Selective hydrogenation of naphthalene over γ-Al2O3-supported NiCu and NiZn bimetal catalysts. Catalysts 10:1215. https://doi.org/10.3390/catal10101215
Feng G, Huo C-F, Deng C-M et al (2009) Isopropanol adsorption on γ-Al2O3 surfaces: a computational study. J Mol Catal A Chem 304:58–64. https://doi.org/10.1016/j.molcata.2009.01.024
Ja Hun K, Jianzhi H, Donghai M et al (2009) Coordinatively unsaturated Al3+ centers as binding sites for active catalyst phases of platinum on gamma-Al2O3. Science 325:1670–1673. https://doi.org/10.1126/science.1176745
Sun G, Alexandrova AN, Sautet P (2019) Pt8 cluster on alumina under a pressure of hydrogen: support-dependent reconstruction from first-principles global optimization. J Phys Chem C 151:194703. https://doi.org/10.1063/1.5129296
Mager-Maury C, Chizallet C, Sautet P, Raybaud P (2012) Platinum nanoclusters stabilized on γ-alumina by chlorine used as a capping surface ligand: a density functional theory study. ACS Catal 2:1346–1357. https://doi.org/10.1021/cs300178y
Gu J, Wang J, Leszczynski J (2021) Single site Fe on the (110) surface of gamma-Al2O3: insights from a DFT study including the periodic boundary approach. Phys Chem Chem Phys 23:7164–7177. https://doi.org/10.1039/d0cp05718e
Feng G, Ganduglia-Pirovano MV, Huo C-F, Sauer J (2018) Hydrogen spillover to copper clusters on hydroxylated γ-Al2O3. J Phys Chem C 122:18445–18455. https://doi.org/10.1021/acs.jpcc.8b03764
Corral Valero M, Raybaud P, Sautet P (2006) Influence of the hydroxylation of γ-Al2O3 surfaces on the stability and diffusion of single Pd atoms: a DFT study. J Phys Chem B 110:1759–1767. https://doi.org/10.1021/jp0554240
Pan Y-X, Liu C-J, Ge Q (2010) Effect of surface hydroxyls on selective CO2 hydrogenation over Ni4/γ-Al2O3: a density functional theory study. J Catal 272:227–234. https://doi.org/10.1016/j.jcat.2010.04.003
Yang T, Ehara M (2017) Probing the electronic structures of Con (n= 1–5) clusters on γ-Al2O3 surfaces using first-principles calculations. Phys Chem Chem Phys 19:3679–3687. https://doi.org/10.1039/C6CP06785A
Khivantsev K, Jaegers NR, Kwak JH, Szanyi J, Kovarik L (2021) Precise identification and characterization of catalytically active sites on the surface of γ-alumina. Angew Chem Int Ed 133:17663–17671. https://doi.org/10.1002/ange.202102106
Kresse G, Furthmüller J (1996) Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput Mater Sci 6:15–50. https://doi.org/10.1016/0927-0256(96)00008-0
Kresse G, Furthmüller J (1996) Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys Rev B 54:11169–11186. https://doi.org/10.1103/PhysRevB.54.11169
Grimme S, Ehrlich S, Goerigk L (2011) Effect of the damping function in dispersion corrected density functional theory. J Comput Chem 32:1456–1465. https://doi.org/10.1002/jcc.21759
Blöchl PE, Först CJ, Schimpl J (2003) Projector augmented wave method: Ab initio molecular dynamics with full wave functions. Bull Mater Sci 26:33–41. https://doi.org/10.1007/BF02712785
Pe B (1994) Projector augmented-wave method. Phys Rev B 50:17953–17979. https://doi.org/10.1103/PhysRevB.50.17953
Berne BJ, Ciccotti G, Coker DF (1998) Classical and quantum dynamics in condensed phase simulations. World Scientific, Singapore
Li J, Zhan X, Zhang Y, Jacobs G, Das T, Davis BH (2002) Fischer-Tropsch synthesis: effect of water on the deactivation of Pt promoted Co/Al2O3 catalysts. Appl Catal, A-Gen 228:203–212. https://doi.org/10.1016/S0926-860X(01)00977-2
Zhang N, Chen X, Chu B, Cao C, Jin Y, Cheng Y (2017) Catalytic performance of Ni catalyst for steam methane reforming in a micro-channel reactor at high pressure. Chem Eng Process 118:19–25. https://doi.org/10.1016/j.cep.2017.04.015
Acknowledgements
This work was supported by the Key Laboratory of High-efficiency Utilization of Coal and Green Chemical Engineering (Grant No. 2022-K19), National Natural Science Foundation of China (Grant Nos. 21875096, 21868016, 22005296 and 21763018), the Foundation of State and the Key Laboratory for Environment and Energy Catalysis of Jiangxi Province (Grant No. 20181BCD40004).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare no conflict of interest.
Additional information
Handling Editor: Pedro Camargo.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, H., Shi, L., Ye, R. et al. Theoretical investigation of high coverage water adsorption on Co and Ni doped γ-Al2O3 surface. J Mater Sci 57, 16710–16724 (2022). https://doi.org/10.1007/s10853-022-07680-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-022-07680-0