Abstract
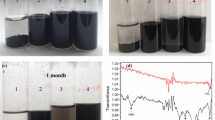
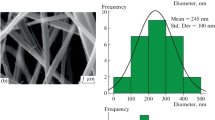
The present study revealed the effect of combining one strong-inorganic and weak-organic protonic acid dopants on the electrical conductivity and electrochemical properties of flexible free-standing composite electrodes based on polyaniline and nanofibrillated cellulose (NFC) or its carboxylated analog (CNFC), synthesized using a bottom-up approach. Hydrochloric acid (HCl) served as a low molecular weight inorganic dopant while phytic acid (PhA) and poly(2-acrylamido-2-methyl-1-propanesulfonic acid) (PAAMPSA) were chosen as the organic dopants. Both PhA and PAAMPSA acted as secondary dopants in the dually doped composites through the molecular conformation changes of PANI chains. Synergistic increase in the electrical conductivity is observed for dually doped PANI-NFC with the combination of PAAMPSA and HCl in comparison with PhA and HCl. Unlike the PhA, the morphological changes induced by PAAMPSA are more favorable for the enhancement of conductivity. Neither the morphological changes, nor the carboxylation of NFC affected the electrochemical properties of the composites as the specific capacitance values were influenced mainly by the type and the strength of the individual acids. The capacitance values per gram of the dually doped composite with PAAMPSA and HCl increased with the decrease in the NFC or CNFC loading reaching values above 200 F·g–1 measured at 50 mV·s−1 of composite for the 80 wt% PANI content. These results highlight the profound impacts of the secondary dopant in PANI on the performance of PANI-based nanocellulose composites.
Graphical Abstract










Similar content being viewed by others
References
Nyholm L, Nystrom G, Mihranyan A, Stromme M (2011) Toward flexible polymer and paper-based energy storage devices. Adv Mater 23(33):3751–3769
Nishide H, Oyaizu K (2008) Toward flexible batteries. Science 319(5864):737–738
Du X, Zhang Z, Liu W, Deng Y (2017) Nanocellulose-based conductive materials and their emerging applications in energy devices–A review. Nano Energy 35:299–320
Moon RJ, Martini A, Nairn J, Simonsen J, Youngblood J (2011) Cellulose nanomaterials review: structure, properties and nanocomposites. Chem Soc Rev 40(7):3941–3994
Siqueira G, Bras J, Dufresne A (2010) Cellulosic bionanocomposites: a review of preparation properties and applications. Polymers 2:728–765
Snook GA, Kao P, Best AS (2011) Conducting-polymer-based supercapacitor devices and electrodes. J Power Sources 196(1):1–12
Yan H, Chunyi Z (2017) Functional flexible and wearable supercapacitors. J Phys D Appl Phys 50(27):273001
Wang Z, Carlsson DO, Tammela P, Hua K, Zhang P, Nyholm L, Strømme M (2015) Surface modified nanocellulose fibers yield conducting polymer-based flexible supercapacitors with enhanced capacitances. ACS Nano 9(7):7563–7571
Zheng W, Lv R, Na B, Liu H, Jin T, Yuan D (2017) Nanocellulose-mediated hybrid polyaniline electrodes for high performance flexible supercapacitors. J Mater Chem A 5(25):12969–12976
Bhandari S, Khastgir D (2015) Synergistic effect of simultaneous dual doping in solvent-free mechanochemical synthesis of polyaniline supercapacitor comparable to the composites with multiwalled carbon nanotube. Polymer 81:62–69
Bhandari S, Singha NK, Khastgir D (2013) Electrochemical synthesis of nanostructured polyaniline: heat treatment and synergistic effect of simultaneous dual doping. J Appl Polym Sci 129(3):1264–1273
MacDiarmid AG, Epstein AJ (1995) Secondary doping in polyaniline. Synth Met 69(1):85–92
Yin W, Ruckenstein E (2000) Soluble polyaniline co-doped with dodecyl benzene sulfonic acid and hydrochloric acid. Synth Met 108(1):39–46
Bavio MA, Acosta GG, Kessler T (2014) Synthesis and characterization of polyaniline and polyaniline–Carbon nanotubes nanostructures for electrochemical supercapacitors. J Power Sources 245:475–481
Singu BS, Srinivasan P, Pabba S (2011) Benzoyl peroxide oxidation route to nano form polyaniline salt containing dual dopants for pseudocapacitor. J Electrochem Soci, 159.
Palaniappan S, Devi SL (2008) Novel chemically synthesized polyaniline electrodes containing a fluoroboric acid dopant for supercapacitors. J Appl Polym Sci 107(3):1887–1892
Arenas MC, Andablo E, Castaño VM (2010) Synthesis of conducting polyaniline nanofibers from single and binary dopant agents. J Nanosci Nanotechnol 10(1):549–554
Stejskal J, Gilbert RG (2002) Polyaniline. Preparation of a conducting polymer(IUPAC technical report). Pure Appl Chem 74(5):857–867
Ma Z, Shi W, Yan K, Pan L, Yu G (2019) Doping engineering of conductive polymer hydrogels and their application in advanced sensor technologies. Chem Sci 10(25):6232–6244
Gawli Y, Banerjee A, Dhakras D, Deo M, Bulani D, Wadgaonkar P, Shelke M, Ogale S (2016) 3D polyaniline architecture by concurrent inorganic and organic acid doping for superior and robust high rate supercapacitor performance. Sci Rep 6:21002
Bautkinová T, Sifton A, Kutorglo EM, Dendisová M, Kopecký D, Ulbrich P, Mazúr P, Laachachi A, Hassouna F (2020) New approach for the development of reduced graphene oxide/polyaniline nanocomposites via sacrificial surfactant-stabilized reduced graphene oxide. Colloids Surf, A Phys Eng Aspects 589:124415
Besbes I, Alila S, Boufi S (2011) Nanofibrillated cellulose from TEMPO-oxidized eucalyptus fibres: effect of the carboxyl content. Carbohyd Polym 84(3):975–983
Trchová M, Morávková Z, Bláha M, Stejskal J (2014) Raman spectroscopy of polyaniline and oligoaniline thin films. Electrochim Acta 122:28–38
Furukawa Y, Ueda F, Hyodo Y, Harada I, Nakajima T, Kawagoe T (1988) Vibrational spectra and structure of polyaniline. Macromolecules 21(5):1297–1305
Jeon JW, Ma Y, Mike JF, Shao L, Balbuena PB, Lutkenhaus JL (2013) Oxidatively stable polyaniline:polyacid electrodes for electrochemical energy storage. Phys Chem Chem Phys 15(24):9654–9662
Vallés C, Jiménez P, Muñoz E, Benito AM, Maser WK (2011) Simultaneous reduction of graphene oxide and polyaniline: doping-assisted formation of a solid-state charge-transfer complex. J Phys Chem C 115(21):10468–10474
Cho S, Lee JS, Jun J, Kim SG, Jang J (2014) Fabrication of water-dispersible and highly conductive PSS-doped PANI/graphene nanocomposites using a high-molecular weight PSS dopant and their application in H2S detection. Nanoscale 6(24):15181–15195
Pouget JP, Jozefowicz ME, Epstein AJ, Tang X, MacDiarmid AG (1991) X-ray structure of polyaniline. Macromolecules 24(3):779–789
French AD (2013) Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 21(2):885–896
Kutorglo EM, Hassouna F, Beltzung A, Kopecký D, Sedlářová I, Šoóš M (2019) Nitrogen-rich hierarchically porous polyaniline-based adsorbents for carbon dioxide (CO2) capture. Chem Eng J 360:1199–1212
Zhang X, Zhu J, Haldolaarachchige N, Ryu J, Young DP, Wei S, Guo Z (2012) Synthetic process engineered polyaniline nanostructures with tunable morphology and physical properties. Polymer 53(10):2109–2120
Chen S-A, Lee H-T (1994) (1994) Structure and properties of poly(acry1ic acid)-doped polyaniline. Macromolecules 28:2858–2866
Arsov LD, Plieth W, Koßmehl G (1998) Electrochemical and Raman spectroscopic study of polyaniline; influence of the potential on the degradation of polyaniline. J Solid State Electrochem 2(5):355–361
Costentin C, Porter TR, Savéant J-M (2017) How do pseudocapacitors store energy? theoretical analysis and experimental illustration. ACS Appl Mater Interfaces 9(10):8649–8658
Posadas D, Rodriguez Presa MJ, Florit MI (2001) Apparent formal redox potential distribution in electroactive arylamine-derived polymers. Electrochim Acta 46(26):4075–4081
Motheo AJ, Santos JR, Venancio EC, Mattoso LHC (1998) Influence of different types of acidic dopant on the electrodeposition and properties of polyaniline films. Polymer 39(26):6977–6982
Li X (2009) Improving the electrochemical properties of polyaniline by co-doping with titanium ions and protonic acid. Electrochim Acta 54(24):5634–5639
Nekrasov AA, Gribkova OL, Ivanov VF, Vannikov AV (2010) Electroactive films of interpolymer complexes of polyaniline with polyamidosulfonic acids: advantageous features in some possible applications. J Solid State Electrochem 14(11):1975–1984
Tarver J, Yoo JE, Dennes TJ, Schwartz J, Loo Y-L (2009) Polymer acid doped polyaniline is electrochemically stable beyond pH 9. Chem Mater 21(2):280–286
Zhang X, Zhang H, Lin Z, Yu M, Lu X, Tong Y (2016) Recent advances and challenges of stretchable supercapacitors based on carbon materials. Sci China Mater 59(6):475–494
Acknowledgements
The authors would like to thank Czech Science Foundation (GAČR No. 21–09830S) for the financial support. We thank also European Regional Development Fund-Project (ORGBAT) No. CZ.02.1.01/0.0/0.0/16_025/0007445 for the financial support of Dr. Mazúr. The authors would like to thank also the Specific University Research (A2_FCHI_2022_007).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Additional information
Handling Editor: Jaime Grunlan.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bautkinová, T., Mazúr, P., Soukupová, G. et al. Tailor-made dual doping for morphology control of polyaniline chains in cellulose nanofiber-based flexible electrodes: electrical and electrochemical performance. J Mater Sci 57, 13945–13961 (2022). https://doi.org/10.1007/s10853-022-07491-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-022-07491-3