Abstract
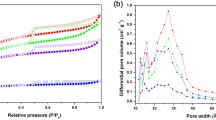
Although carbon material are widely used for gas separation and adsorption due to its well-developed pore structure, their prevalent wide pore size distribution and scarcity of active adsorption sites limit its gas adsorption capacity. Therefore, in this paper, a series of N-doped microporous carbon adsorbent materials were prepared via utilizing cheap starch as the carbon source and the synthesized melamine resin as the N-doped modifier with the assistance of hydrothermal conversion and high temperature activation by KOH.BET test showed that MF@Cs was a typical microporous carbon material with a pore size distribution of 0.3–2 nm, and its most accessible pore size was about 0.6 nm,which benefits the adsorption of CO2 and CH4. MF@C–1–750 porous carbon material present prominent pore structure parameters, with a maximum specific surface area of 2415.9 cm2/g, a total pore volume of 1.36 cm2/g, and a maximum ultramicro pore volume of 0.42 cm2/g at 0.3 ~ 1.0 nm. MF@Cs porous carbon materials show high static adsorption capacity for CO2 and CH4, the adsorption capacity of CO2 of MF@C–1–750 is as high as 6.54 mmol/g at 273 K and 100 kPa, which is attributed to the excellent ultramicro pore volume of carbon materials. MF@Cs porous carbon material is expected to play a huge application potential in the separation and enrichment of CO2 in the future, due to its simple preparation and low cost, excellent specific surface area, outstanding ultrafine pore capacity and high adsorption capacity of CO2 gas.









Similar content being viewed by others
References
Jain A, Balasubramanian R, Srinivasan MP (2016) Hydrothermal conversion of biomass waste to activated carbon with high porosity: a review. Chem Eng J 283:789
Zhong D-L, Wang W-C, Zou Z-L, Lu Y-Y, Yan J, Ding K (2018) Investigation on methane recovery from low-concentration coal mine gas by tetra-n-butyl ammonium chloride semiclathrate hydrate formation. Appl Energy 227:686
Li L, Yang L, Wang J, Zhang Z, Yang Q, Yang Y, Ren Q, Bao Z (2018) Highly efficient separation of methane from nitrogen on a squarate-based metal-organic framework. AlChE J 64:3681
Niu Z, Cui X, Pham T, Lan PC, Xing H, Forrest KA, Wojtas L, Space B, Ma S (2019) A metal-organic framework based methane nano-trap for the capture of coal-mine methane. Angew Chem Int Ed Engl 58:10138–10141
Xu S, Li WC, Wang CT, Tang L, Hao GP, Lu AH (2021) Self-pillared ultramicroporous carbon nanoplates for selective separation of CH4 /N2. Angew Chem Int Ed Engl 60:6339–6343
Xue CL, Cheng WP, Hao WM, Ma JH, Li RF (2019) CH4/N2 Adsorptive Separation on Zeolite X/AC Composites. J Chem 2019:1
Hao X, Li Z, Hu H, Liu X, Huang Y (2018) Separation of CH4/N2 of low concentrations from coal bed gas by sodium-modified clinoptilolite. Front Chem 6:663
Hung T-H, Deng X, Lyu Q, Lin L-C, Kang D-Y (2021) Coulombic effect on permeation of CO2 in metal-organic framework membranes. J Membr Sci 639:119742
Matei Ghimbeu C, Górka J, Simone V, Simonin L, Martinet S, Vix-Guterl C (2018) Insights on the Na+ ion storage mechanism in hard carbon: Discrimination between the porosity, surface functional groups and defects. Nano Energy 44:327
Kiljanek T, Niewiadowska A, Malysiak M, Posyniak A (2021) Miniaturized multiresidue method for determination of 267 pesticides, their metabolites and polychlorinated biphenyls in low mass beebread samples by liquid and gas chromatography coupled with tandem mass spectrometry. Talanta 235:122721
Huang G, Wang Y, Zhang T, Wu X, Cai J (2019) High-performance hierarchical N-doped porous carbons from hydrothermally carbonized bamboo shoot shells for symmetric supercapacitors. J Taiwan Inst Chem Eng 96:672
Yoshikawa M, Katagiri G, Ishida H, Ishitani A, Akamatsu T (1988) Resonant Raman scattering of diamondlike amorphous carbon films. Appl Phys Lett 52:1639
Liang Y, Tan Y, Wang F, Luo Y, Zhao Z (2020) Improving permeability of coal seams by freeze-fracturing method: The characterization of pore structure changes under low-field NMR. Energy Rep 6:550
Manmuanpom N, Thubsuang U, Dubas ST, Wongkasemjit S, Chaisuwan T (2018) Enhanced CO2 capturing over ultra-microporous carbon with nitrogen-active species prepared using one-step carbonization of polybenzoxazine for a sustainable environment. J Environ Manage 223:779
Zhang P, Wang J, Fan W, Zhong Y, Zhang Y, Deng Q, Zeng Z, Deng S (2019) Ultramicroporous carbons with extremely narrow pore size distribution via in-situ ionic activation for efficient gas-mixture separation. Chem Eng J 375:121931
Tang R, Dai Q, Liang W, Wu Y, Zhou X, Pan H, Li Z (2020) Synthesis of novel particle rice-based carbon materials and its excellent CH4/N2 adsorption selectivity for methane enrichment from Low-rank natural gas. Chem Eng J 384:123388
Li Y, Wang S, Wang B, Wang Y, Wei J (2020) Sustainable biomass glucose-derived porous carbon spheres with high nitrogen doping: as a promising adsorbent for CO2/CH4/N2 adsorptive separation. Nanomaterials 10:174
Liu F, Zhang Y, Zhang P, Xu M, Tan T, Wang J, Deng Q, Zhang L, Wan Y, Deng S (2020) Facile preparation of N and O-rich porous carbon from palm sheath for highly selective separation of CO2/CH4/N2 gas-mixture. Chem Eng J 399:125812
Liu S, Yang P, Wang L, Li Y, Wu Z, Ma R, Wu J, Hu X (2019) Nitrogen-doped porous carbons from lotus leaf for CO2 capture and supercapacitor electrodes. Energy Fuels 33:6568
Nie Z, Huang Y, Ma B, Qiu X, Zhang N, Xie X, Wu Z (2019) Nitrogen-doped carbon with modulated surface chemistry and porous structure by a stepwise biomass activation process towards enhanced electrochemical lithium-ion storage. Sci Rep 9:15032
Ren X, Li H, Chen J, Wei L, Modak A, Yang H, Yang Q (2017) N-doped porous carbons with exceptionally high CO2 selectivity for CO2 capture. Carbon 114:473
Saha D, Van Bramer SE, Orkoulas G, Ho H-C, Chen J, Henley DK (2017) CO2 capture in lignin-derived and nitrogen-doped hierarchical porous carbons. Carbon 121:257
Wang L, Rao L, Xia B, Wang L, Yue L, Liang Y, DaCosta H, Hu X (2018) Highly efficient CO2 adsorption by nitrogen-doped porous carbons synthesized with low-temperature sodium amide activation. Carbon 130:31
Zhang Y, Liu L, Zhang P, Wang J, Xu M, Deng Q, Zeng Z, Deng S (2019) Ultra-high surface area and nitrogen-rich porous carbons prepared by a low-temperature activation method with superior gas selective adsorption and outstanding supercapacitance performance. Chem Eng J 355:309
Zhong DL, Wang WC, Zou ZL, Lu YY, Yan J, Ding K (2018) Investigation on methane recovery from low-concentration coal mine gas by tetra-n-butyl ammonium chloride semiclathrate hydrate formation. Appl Energy Bark Then Oxford 686:693
Hu B, Liu JT, Chen CJ, Zhao Z, Chang SJ, Kang PL (2017) Ultra-low charge transfer resistance carbons by one-pot hydrothermal method for glucose sensing. Sci China Mater 60:1234
Zhukavin RK, Kovalevskii KA, Sergeev SM, Choporova YY, Gerasimov VV, Tsyplenkov VV, Knyazev BA, Abrosimov NV, Pavlov SG, Shastin VN, Schneider H, Deßmann N, Shevchenko OA, Vinokurov NA, Kulipanov GN, Hübers H-W (2017) Low-temperature intracenter relaxation times of shallow donors in germanium. JETP Lett 106:571–575
Yang Z, Guo X, Zhang G, Xu Y (2020) One-pot synthesis of high N-doped porous carbons derived from a N-rich oil palm biomass residue in low temperature for CO 2 capture. Int J Energy Res 44:4875–4887
Yuan M, Que H, Yang X, Li M (2019) Nitrogen and oxygen co-doped glucose-based carbon materials with enhanced electrochemical performances as supercapacitors. Ionics 25:4305–4315
Yang C, Zhao T, Pan H, Liu F, Cao J, Lin Q (2021) Facile preparation of N -doped porous carbon from chitosan and NaNH 2 for CO 2 adsorption and conversion. Chem Eng J 283:789
Yan Y, Chaudhuri SR, Sarkar A (1996) Synthesis of oriented zeolite molecular sieve films with controlled morphologies. Chem Mater 8:473–479
Yafei G, Chang T, Jian S, Weiling L, Jubing Z (2022) Porous activated carbons derived from waste sugarcane bagasse for CO2 adsorption—ScienceDirect. Chem Eng J 433:133680
Wfab C, Zhab C, Yang Z, Pei S, Qzab C, Yjab C, Mzab C (2021) Tuning mobility of intermediate and electron transfer to enhance electrochemical reduction of nitrate to ammonia on Cu 2 O/Cu interface. Chem Eng J 381:122736
Cavenati S, Grande C, Rodrigues A (2005) Separation of methane and nitrogen by adsorption on carbon molecular sieve. Sep Sci Technol 40:2721
Chen A, Li S, Yu Y, Liu L, Li Y, Wang Y, Xia K (2016) Self-catalyzed strategy to form hollow carbon nanospheres for CO2 capture. Mater Lett 185:63–66
Geng L, Zhou W, Wang X, Li T, Nowak AP, Liu Z, Zhang Y-Z, Zhang D-S, Zhang X, Han H (2021) Redox property switching in MOFs with open metal sites for improved catalytic hydrogenation performance. J Alloys Compd 888:161494
Liu J, Fan Y-Z, Zhang K, Zhang L, Su C-Y (2020) Engineering porphyrin metal-organic framework composites as multifunctional platforms for CO2 adsorption and activation. J Am Chem Soc 142:14548
Psarras P, Holmes R, Vishal V, Wilcox J (2017) Methane and CO2 adsorption capacities of kerogen in the eagle ford shale from molecular simulation. Acc Chem Res 50:1818–1828
Acknowledgements
This work was supported by Nature Science Foundation of China (Grant 22068009), Science and Technology Support Plan Projects of Guizhou Province (Grant (2018) 2192), Scientific and Technological Innovation Talents Team of Guizhou (2018-5607), Science and Technology Foundation of Guizhou Province (2017-7254), One hundred Person Project of Guizhou Province (No.20165655), Innovation Group Project of Education Department in Guizhou Province (No. 2021010).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Handling Editor: Annela M. Seddon.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dang, W., Lin, Q., Pan, H. et al. Facile preparation of N-doped porous carbon and its CO2 gas adsorption performance. J Mater Sci 57, 12438–12448 (2022). https://doi.org/10.1007/s10853-022-07409-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-022-07409-z