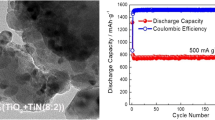
Abstract
As the demand for higher-performance batteries has increased, so has the body of research on theoretical high-capacity anode materials. However, the research has been hindered because the high-capacity anode material properties and interactions are not well understood, largely due to the difficulty of observing cycling in situ. Using electrochemical scanning transmission electron microscopy (ec-STEM), we report the real-time observation and electrochemical analysis of pristine tin (Sn) and titanium dioxide-coated Sn (TiO2@Sn) electrodes during lithiation/delithiation. As expected, we observed a volume expansion of the pristine Sn electrodes during lithiation, but we further observed that the expansion was followed by Sn detachment from the current collector. Remarkably, although the TiO2@Sn electrodes also exhibited similar volume expansion during lithiation, they showed no evidence of Sn detachment. We found that the TiO2 surface layer acted as an electrochemically activated artificial solid-electrolyte interphase that serves to conduct Li ions. As a physical coating, it mechanically prevented Sn detachment following volume changes during cycling, providing significant degradation resistance and 80% Coulombic efficiency for a complete lithiation/delithiation cycle. Interestingly, upon delithiation, TiO2@Sn electrode displayed a self-healing mechanism of small pore formation in the Sn particle followed by agglomeration into several larger pores as delithiation continued.
Graphical abstract





Similar content being viewed by others
References
Schmuch R, Wagner R, Hörpel G, Placke T, Winter M (2018) Performance and cost of materials for lithium-based rechargeable automotive batteries. Nat Energy 3:267–278
Albertus P, Babinec S, Litzelman S, Newman A (2018) Status and challenges in enabling the lithium metal electrode for high-energy and low-cost rechargeable batteries. Nat Energy 3:16–21
Li M, Lu J, Chen Z, Amine K (2018) 30 Years of lithium-ion batteries. Adv Mater 30:1800561
Liu L, Xie F, Lyu J, Zhao T, Li T, Choi BG (2016) Tin-based anode materials with well-designed architectures for next-generation lithium-ion batteries. J Power Sour 321:11–35
Li W, Sun X, Yu Y (2017) Si-, Ge-, Sn-based anode materials for lithium-ion batteries: from structure design to electrochemical performance. Small Methods 1:1600037
Liu D, Liu ZJ, Li X, Xie W, Wang Q, Liu Q, Fu Y, He D (2017) Group IVA element (Si, Ge, Sn)-based alloying/dealloying anodes as negative electrodes for full-cell lithium-ion batteries. Small 13:1702000
Wachtler M, Besenhard JO, Winter M (2001) Tin and tin-based intermetallics as new anode materials for lithium-ion cells. J Power Sour 94:189–193
Chou C-Y, Kim H, Hwang GS (2011) A comparative first-principles study of the structure, energetics, and properties of Li–M (M = Si, Ge, Sn) alloys. J Phys Chem C 115:20018–20026
Goriparti S, Miele E, De Angelis F, Di Fabrizio E, Proietti Zaccaria R, Capiglia C (2014) Review on recent progress of nanostructured anode materials for Li-ion batteries. J Power Sour 257:421–443
Wang J, Fan F, Liu Y, Jungjohann KL, Lee SW, Mao SX, Liu X, Zhu T (2014) Structural evolution and pulverization of Tin nanoparticles during lithiation-delithiation cycling. J Electrochem Soc 161:F3019–F3024
Li Q, Wang P, Feng Q, Mao M, Liu J, Mao SX, Wang H (2014) In Situ TEM on the reversibility of nanosized Sn anodes during the electrochemical reaction. Chem Mater 26:4102–4108
Leenheer AJ, Jungjohann KL, Zavadil KR, Harris CT (2016) Phase boundary propagation in Li-alloying battery electrodes revealed by liquid-cell transmission electron microscopy. ACS Nano 10:5670–5678
Wu F, Yao N (2015) Advances in sealed liquid cells for in-situ TEM electrochemial investigation of lithium-ion battery. Nano Energy 11:196–210
Zeng Z, Liang W-I, Liao H-G, Xin HL, Chu Y-H, Zheng H (2014) Visualization of electrode-electrolyte interfaces in LiPF6/EC/DEC electrolyte for lithium ion batteries via in situ TEM. Nano Lett 14:1745–1750
Leenheer AJ, Jungjohann KL, Zavadil KR, Sullivan JP, Harris CT (2015) Lithium electrodeposition dynamics in aprotic electrolyte observed in situ via transmission electron microscopy. ACS Nano 9:4379–4389
Gu M, Parent LR, Mehdi BL, Unocic RR, McDowell MT, Sacci RL, Xu W, Connell JG, Xu P, Abellan P et al (2013) Demonstration of an electrochemical liquid cell for operando transmission electron microscopy observation of the lithiation/delithiation behavior of Si nanowire battery anodes. Nano Lett 13:6106–6112
Mackay DT, Janish MT, Sahaym U, Kotula PG, Jungjohann KL, Carter CB, Norton MG (2014) Template-free electrochemical synthesis of tin nanostructures. J Mater Sci 49:1476–1483. https://doi.org/10.1007/s10853-013-7917-1
Harrison KL, Zavadil KR, Hahn NT, Meng X, Elam JW, Leenheer A, Zhang J-G, Jungjohann KL (2017) Lithium self-discharge and its prevention: direct visualization through in situ electrochemical scanning transmission electron microscopy. ACS Nano 11:11194–11205
Guan C, Wang X, Zhang Q, Fan Z, Zhang H, Fan HJ (2014) Highly stable and reversible lithium storage in SnO2 Nanowires surface coated with a uniform hollow shell by atomic layer deposition. Nano Lett 14:4852–4858
Chen J, Yang L, Zhang Z, Fang S, Hirano SI (2013) Mesoporous TiO2–Sn@ C core–shell microspheres for Li-ion batteries. Chem Commun 49:2792–2794
Su N, Li L, Junfang L, Jing X, Yue Z, Jianjun X, Min L, Xianyou W (2019) TiO2-Sn/C composite nanofibers with high-capacity and long-cycle life as anode materials for sodium ion batteries. J Alloy Compd 772:314–323
Moitzheim S, De Gendt S, Vereecken PM (2019) Investigation of the Li-ion insertion mechanism for amorphous and anatase TiO2 thin-films. J Electrochem Soc 166:A1–A9
Noh M, Kwon Y, Lee H, Cho J, Kim Y, Kim MG (2005) Amorphous carbon-coated Tin anode material for lithium secondary battery. Chem Mater 17:1926–1929
Hu T, Xie M, Zhong J, Sun HT, Sun X, Scott S, Lian J (2014) Porous Fe2O3 nanorods anchored on nitrogen-doped graphenes and ultrathin Al2O3 coating by atomic layer deposition for long-lived lithium ion battery anode. Carbon 76:141–147
Zhu G-N, Wang Y-G, Xia Y-Y (2012) Ti-based compounds as anode materials for Li-ion batteries. Energy Environ Sci 5:6652–6667
Goriparti S, Miele E, Prato M, Scarpellini A, Marras S, Monaco S, Toma A, Messina GC, Alabastri A, Angelis FD et al (2015) Direct synthesis of carbon-doped TiO2–bronze nanowires as anode materials for high performance lithium-ion batteries. ACS Appl Mater Interfaces 7:25139–25146
Bruce PG, Scrosati B, Tarascon J-M (2008) Nanomaterials for rechargeable lithium batteries. Angew Chem Int Ed 47:2930–2946
Armstrong AR, Armstrong G, Canales J, Bruce PG (2005) TiO2–B nanowires as negative electrodes for rechargeable lithium batteries. J Power Sources 146:501–506
Wagemaker M, Kearley GJ, van Well AA, Mutka H, Mulder FM (2003) Multiple Li positions inside oxygen octahedra in lithiated TiO2 anatase. J Am Chem Soc 125:840–848
Gao X, Sun X, Jiang Z, Wang Q, Gao N, Li H, Zhang H, Yu K, Su C (2019) Introducing nanodiamond into TiO2-based anode for improving the performance of lithium-ion batteries. New J Chem 43:3907–3912
Leenheer AJ, Sullivan JP, Shaw MJ, Harris CT (2015) A sealed liquid cell for in situ transmission electron microscopy of controlled electrochemical processes. J Microelectromech Syst 24:1061–1068
Elofsson V, Lü B, Magnfält D, Münger EP, Sarakinos K (2014) Unravelling the physical mechanisms that determine microstructural evolution of ultrathin Volmer-Weber films. J Appl Phys 116:044302
Wei H, Eilers H (2009) From silver nanoparticles to thin films: evolution of microstructure and electrical conduction on glass substrates. J Phys Chem Sol 70:459–465
Memarzadeh Lotfabad E, Kalisvaart P, Cui K, Kohandehghan A, Kupsta M, Olsen B, Mitlin D (2013) ALD TiO2 coated silicon nanowires for lithium ion battery anodes with enhanced cycling stability and coulombic efficiency. Phys Chem Chem Phys 15:13646–13657
Lotfabad EM, Kalisvaart P, Kohandehghan A, Cui K, Kupsta M, Farbod B, Mitlin D (2014) Si nanotubes ALD coated with TiO2, TiN or Al2O3 as high performance lithium ion battery anodes. J Mater Chem A 2:2504–2516
Jeong G, Kim J-H, Kim Y-U, Kim Y-J (2012) Multifunctional TiO2 coating for a SiO anode in Li-ion batteries. J Mater Chem 22:7999–8004
Shen BH, Veith GM, Tenhaeff WE (2018) Silicon surface tethered polymer as artificial solid electrolyte interface. Sci Rep 8:11549
Menkin S, Golodnitsky D, Peled E (2009) Artificial solid-electrolyte interphase (SEI) for improved cycleability and safety of lithium-ion cells for EV applications. Electrochem Commun 11:1789–1791
Liu P, Wang S, Li D, Li Y, Chen X-Q (2016) Fast and Huge anisotropic diffusion of Cu (Ag) and its resistance on the Sn self-diffusivity in solid β-Sn. J Mater Sci Technol 32:121–128
Boas W, Fensham PJ (1949) Rate of self-diffusion in Tin crystals. Nature 164:1127–1128
Lu X, He Y, Mao SX, Wang CM, Korgel BA (2016) Size dependent pore formation in germanium nanowires undergoing reversible delithiation observed by in situ TEM. J Phys Chem C 120(50):28825–28831
Lu X, Bogart TD, Gu M, Wang C, Korgel BA (2015) In situ TEM observations of Sn-containing silicon nanowires undergoing reversible pore formation due to fast lithiation/delithiation kinetics. J Phys Chem C 119:21889–21895
Adkins ER, Jiang T, Luo L, Wang C-M, Korgel BA (2018) In situ transmission electron microsopy of oxide shell-induced pore formation in (De)lithiated silicon nanowires. ACS Energy Lett 3:2829–2834
Shen C, Ge M, Luo L, Fang X, Liu Y, Zhang A, Rong J, Wang C, Zhou C (2016) In situ and ex situ TEM study of lithiation behaviours of porous silicon nanostructures. Sci Rep 6:31334
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Additional information
Handling Editor: Mark Bissett.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10853_2021_6265_MOESM1_ESM.docx
HAADF images, EDS mapping of TiO2@Sn electrode, Coulombic efficiency vs cycle number of pristine Sn and TiO2@Sn electrodes, additional replicate experiments, and estimate of volume changes after lithiation of TiO2@Sn electrode (DOCX 4432 kb)
Full pristine Sn electrode lithiation with grain size about 50–100 nm at current 1 mA/cm2 (AVI 4113 kb)
Full pristine Sn electrode lithiation with grain size about 500 nm-1 µm at current 1 mA/cm2 (AVI 150 kb)
Full TiO2@Sn electrode lithiation grain size about 500 nm-1 µm at current 1 mA/cm2 (AVI 42 kb)
Full TiO2@Sn electrode delithiation grain size about 500 nm-1 µm at current 1 mA/cm2 (AVI 29 kb)
Rights and permissions
About this article
Cite this article
Goriparti, S., Harrison, K.L. & Jungjohann, K.L. Degradation-resistant TiO2@Sn anodes for high-capacity lithium-ion batteries. J Mater Sci 56, 17156–17166 (2021). https://doi.org/10.1007/s10853-021-06265-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-021-06265-7