Abstract
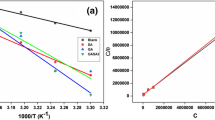
Sustainable betalain pigments extracted from beetroots were used as eco-friendly film coating over aluminium surface. The film coating was examined and verified by corrosive 1.0 M HCl solution. Characterization of chemical structure of the betalain pigments was done using spectroscopic attenuated total reflectance Fourier transform infrared (ATR-FTIR) and UV–Vis technique. Scanning electron microscope technique was used to demonstrate the eco-friendly film coating before and after been examined via corrosive 1.0 M HCl solution. Various operating conditions were studied to meet highest inhibition efficiency values such as betalain concentration, operating temperature and turbulent flow, and maximum inhibition efficiency = 98.4% was displayed. Multiple adsorption isotherms like Langmuir, Temkin, and Freundlich were used effectively to determine equilibrium constants and adsorption parameters. Dynamic investigations revealed a decline of rate of corrosion (RC) and rise of activation energy values (Ea) at higher betalain concentrations. Thermodynamic investigations revealed physisorption process, exothermic enthalpy, and low entropic values of betalain on aluminium surface. The noticeable specifications of low cost, abundance and sustainability, environmentally friendly, and high technical feasibility of betalain pigments adapt them to be the futuristic outstanding sustainable film coating for metal surfaces.
Graphical abstract






Similar content being viewed by others
References
Chaubey N, Savita AQ, Chauhan DS, Quraishi MA (2021) Frontiers and advances in green and sustainable inhibitors for corrosion applications: a critical review. J Mol Liq 321:114385
Ahanotu CC, Onyeachu IB, Solomon MM, Chikwe IS, Chikwe OB, Eziukwu CA (2020) Pterocarpus santalinoides leaves extract as a sustainable and potent inhibitor for low carbon steel in a simulated pickling medium. Sustain Chem Pharm 15:100196
Arzt E, Quan H, McMeeking RM, Hensel R (2021) Functional surface microstructures inspired by nature – From adhesion and wetting principles to sustainable new devices. Prog Mater Sci 119:100778
Saeed MT, Saleem M, Niyazi AH et al (2020) Carrot (Daucus Carota L.) peels extract as an herbal corrosion inhibitor for mild steel in 1M HCl solution. Mod Appl Sci 14(2):97–112
Salleh SZ, Yusoff AH, Zakaria SK, Taib MAA, Seman AA, Masri MN, Mohamad M, Mamat S, Sobri SA, Ali A, Teo PT (2021) Plant extracts as green corrosion inhibitor for ferrous metal alloys: A review. J Cleaner Prod 304:127030
Addi BA, Addi AA, Shaban A, Addi EHA, Hamdani M (2021) Comparative electrochemical and spectroscopic analysis of the inhibition effect of molybdates and boiled red onion extract on the corrosion of tin in acidic solution. J Adhes Sci Technol 35(8):856–872. https://doi.org/10.1080/01694243.2020.1826827
Al-Akhras N, Mashaqbeh Y (2021) Potential use of eucalyptus leaves as green corrosion inhibitor of steel reinforcement. J Build Eng 35:101848
Ashassi-Sorkhabi H, Es’haghi M (2009) Corrosion inhibition of mild steel in hydrochloric acid by betalain as a green inhibitor. J Solid State Electrochem 13(8):1297–1301
Alejandra Guerrero-Rubio M, Hernández-García Samanta, García-Carmona Francisco, Gandía-Herrero Fernando (2021) Biosynthesis of a novel polymeric chitosan-betaxanthin and characterization of the first sugar-derived betalains and their effects in the in vivo model caenorhabditis elegans. Carbohydr Polym 252:117141
Das Trishitman PS, Negi NK Rastogi (2021) Concentration of beetroot juice colorant (betalains) by forward osmosis and its comparison with thermal processing. LWT 145:111522
Montiel-Sánchez M, García-Cayuela T, Gómez-Maqueo A, García HS, Pilar Cano M (2021) In vitro gastrointestinal stability, bioaccessibility and potential biological activities of betalains and phenolic compounds in cactus berry fruits (Myrtillocactus geometrizans). Food Chem 342:128087
Sharma R, Oberoi HS, Dhillon GS (2016) Fruit and vegetable processing waste: renewable feed stocks for enzyme production. In: Dhillon S, Kaur S (eds) Agro-industrial wastes as feedstock for enzyme production. Academic Press, pp 23–59
Ravichandran K, Saw NMMT, Mohdaly AAA, Gabr AMM, Kastell A, Riedel H, Cai Z, Knorr D, Smetanska I (2013) Impact of processing of red beet on betalain content and antioxidant activity. Food Res Int 50(2):670–675
Sutariya B, Saraf M (2017) Betanin, isolated from fruits of opuntia elatior Mill attenuates renal fibrosis in diabetic rats through regulating oxidative stress and TGF-β pathway. J Ethnopharmacol 198(23):432–443
Li S, Bin Mu, Wang X, Kang Y, Wang A (2020) Fabrication of eco-friendly betanin hybrid materials based on Palygorskite and Halloysite. Materials 13:4649
Zhang D, Lanier SM, Downing JA, Avent JL, Lum J, McHale JL (2008) Betalain pigments for dye-sensitized solar cells. J Photochem Photobiol A 195:72–80
Quin C, Clark AE (2007) DFT characterization of the optical and redox properties of natural pigments relevant to dye-sensitized solar cells. Chem Phys Lett 438:26–30
Calogero G, Di Marco G, Cazzanti S, Caramori S, Argazzi R, Di Carlo A, Alberto Bignozzi C (2010) Efficient dye-sensitized solar cells using red turnip and purple wild Sicilian prickly pear fruits. Inter J Mol Sci 11:254–267
García-Salinas MJ, Ariza MJ (2019) Optimizing a simple natural dye production method for dye-sensitized solar cells: examples for betalain (bougainvillea and beetroot extracts) and anthocyanin dyes. Appl Sci 9:2515
Belakhdar A, Ferkous H, Djellali S, Sahraoui R, Lahbib H, Amor YB, Erto A, Balsamo M, Benguerba Y (2020) Computational and experimental studies on the efficiency of Rosmarinus officinalis polyphenols as green corrosion inhibitors for XC48 steel in acidic medium. Coll Surf A: Physicochem Eng Asp 606:125458
Zaher A, Chaouiki A, Salghi R, Boukhraz A, Bourkhiss B, Ouhssine M (2020) Inhibition of mild steel corrosion in 1M hydrochloric medium by the methanolic extract of Ammi Visnaga L. lam seeds. Int J Corros 2020:1–10
Farahati R, Morteza Mousavi-Khoshdel S, Ghaffarinejad A, Behzadi H (2020) Experimental and computational study of penicillamine drug and cysteine as water-soluble green corrosion inhibitors of mild steel. Progress Organ Coat 142:105567
Zhao Q, Guo J, Cui G, Han T, Wu Y (2020) Chitosan derivatives as green corrosion inhibitors for P110 steel in a carbon dioxide environment. Colloids Surf B Biointerfaces 194:111150. https://doi.org/10.1016/j.colsurfb.2020.111150
Chung I-M, Malathy R, Kim S-H, Kalaiselvi K, Prabakaran M, Gopiraman M (2020) Ecofriendly green inhibitor from Hemerocallis fulva against aluminum corrosion in sulphuric acid medium. J Adhes Sci Technol 34(14):1–24
Fares MM, Masadeh KH (2015) Glutamine-reinforced silica gel microassembly as protective coating for aluminium surface. Mater Chem Phys 162:124–130
Nathiya RS, Perumal S, Murugesan V, Raj V (2019) Evaluation of extracts of Borassus flabellifer dust as green inhibitors for aluminium corrosion in acidic media. Mater Sci Semicond Process 104:104674
Obat IB, Onyeachu OB, Umoren SA (2019) Alternative corrosion inhibitor formulation for carbon steel in CO2-saturated brine solution under high turbulent flow condition for use in oil and gas transportation pipelines. Corros Sci 159:108140
Laidler K, Meiser J, Sanctuary B (2000) Physical chemistry, 4th edn. Haughton Mifflin company, NY, pp 933–938
Rashidi NA, Yusupa S, Borhan A (2016) Isotherm and thermodynamic analysis of carbon dioxide on activated carbon. Procedia Eng 148:630–637
Ituen EB, Akaranta O, James OA (2015) 5-Hydroxytryptophan: a novel eco-friendly corrosion and oilfield microbial inhibitor. Soc Pet Eng. https://doi.org/10.2118/178370-MS
Fares MM, Maayta AK, Al-Mustafa JA (2013) Synergistic corrosion inhibition of aluminum by polyethylene glycol and ciprofloxacin in acidic media. J Adhes Sci Technol 27(23):2495–2506
Gerengi H, Schaefer K, Sahin HI (2012) Corrosion-inhibiting effect of Mimosa extract on brass-MM55 corrosion in 0.5 M H2SO4 acidic media. J Ind Eng Chem 18:2204–2210
Fares MM, Maayta AK, Al-Qudah MA (2013) Polysorbate20 adsorption layers below and above the critical micelle concentration over aluminum; cloud point and inhibitory role investigations at the solid/liquid interface. Surf Interface Anal 45:906–912
OZa BN, Sinha RS (1982) Thermometric study of corrosion behaviour of high strength Al-Mg alloy in phosphoric acid in presence of halides. Tanstact Saest 17(4):281–285
Umoren SA, Ogbobe O, Igwe IO, Ebenso EE (2008) Inhibition of mild steel corrosion in acidic medium using synthetic and naturally occurring polymers and synergistic halide additives. Corros Sci 50:1998–2006
Yonghong Wu (2017) The removal of methyl orange by periphytic biofilms: equilibrium and kinetic modeling. In: Periphyton. Functions and application in environmental remediation. Elsevier, pp 367–387. https://doi.org/10.1016/B978-0-12-801077-8.00016-8
Ogunleye OO, Arinkoola AO, Eletta OA, Agbede OO, Osho YA, Morakinyo AF, Hamed JO (2020) Green corrosion inhibition and adsorption characteristics of Luffa cylindrica leaf extract on mild steel in hydrochloric acid environment. Heliyon 6(1):e03205
Herrag L, Hammouti B, Elkadiri S, Aouniti A, Jama C, Vezin H, Bentiss F (2010) Adsorption properties and inhibition of mild steel corrosion in hydrochloric solution by some newly synthesized diamine derivatives: experimental and theoretical investigations. Corros Sci 52:3042–3051
Aslam R, Mobin M, Aslam J, Lgaz H (2018) Sugar based N,N′-didodecyl-N,N′ digluconamideethylenediamine gemini surfactant as corrosion inhibitor for mild steel in 3.5% NaCl solution-effect of synergistic KI additive. Sci Rep 8:3690. https://doi.org/10.1038/s41598-018-21175-6
Fares MM, Maayta AK, Al-Qudah MM (2012) Pectin as promising green corrosion inhibitor of aluminum in hydrochloric acid solution. Corros Sci 60:112–117
Outirite M, Lagrenée M, Lebrini M, Traisnel M, Jama C, Vezin H, Bentiss F (2010) AC impedance, X-ray photoelectron spectroscopy and density functional theory studies of 3,5-bis(n-pyridyl)-1,2,4-oxadiazoles as efficient corrosion inhibitors for carbon steel surface in hydrochloric acid solution. Electrochim Acta 55:1670–1681
Fares MM, Maayta AK, Al-Mustafa JA (2012) Corrosion inhibition efficacy of iota-carrageenan natural polymer on aluminum in presence of zwitterion mediator in HCl media. Corros Sci 65:223–230
Dehri I, Ozcan M (2006) The effect of temperature on the corrosion of mild steel in acidic media in the presence of some sulphur-containing organic compounds. Mater Chem Phys 98:316–323
Wei W, Liu Z, Liang C, Han G-C, Han J, Zhang S (2020) Synthesis, characterization and corrosion inhibition behavior of 2-aminofluorene bis-Schiff bases in circulating cooling water. RSC Adv 10:17816
Ma Y, Han F, Li Z, Xia C (2016) corrosion behavior of metallic materials in acidic-functionalized ionic liquids. ACS Sustain Chem Eng 4(2):633–639
Chugh B, Singh AK, Thakur S, Pani B, Lgaz H, Chung I-M, Jha R, Ebenso EE (2020) comparative investigation of corrosion-mitigating behavior of thiadiazole-derived bis-schiff bases for mild steel in acid medium: experimental Theoretical, and Surface Study. ACS Omega 5(23):13503–13520
Acknowledgements
Authors wish to acknowledge Jordan University for Science & Technology, Deanship of Research, Project Number 20200117 for financial support and facilities.
Funding
This study was funded by Jordan University for Science & Technology, Deanship of Research (Grant Number = 20200117).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Author M.M. Fares has received research grant from Jordan University for Science & Technology. The authors declare that they have no conflict of interest.
Additional information
Handling Editor: M. Grant Norton.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fares, M.M., Bani-Domi, A. Sustainable betalain pigments as eco-friendly film coating over aluminium surface. J Mater Sci 56, 13556–13567 (2021). https://doi.org/10.1007/s10853-021-06179-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-021-06179-4