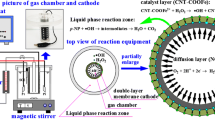
Abstract
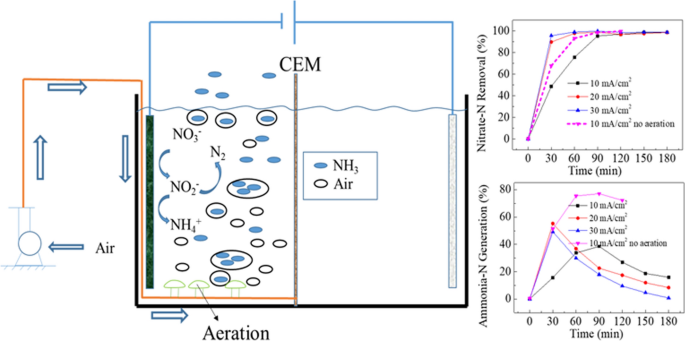
A three-dimensional bimetal carbon-based electrode, CuNi/multi-walled carbon nanotube/graphite felt (CuNi/M/GF), was designed for the electrochemical reduction of nitrate. Multi-walled carbon nanotubes (MWCNTs) served as the interlayer and skeleton, which increased the electrochemical reduction activity of GF to nitrate. CuNi/M/GF with different atomic ratios of copper and nickel was prepared by adjusting the electrodeposition potential. The performance of the CuNi/M/GF, Cu foam, Ni foam, M/GF and GF in a dual-chamber cell and single-chamber cell was evaluated for different concentrations of chlorine, current density, energy consumption and degradation. In a solution of 100 mg/L NO3−-N, the removal of NO3−-N and total nitrogen (TN) by the optimal electrode in a single-chamber cell (SCC) can reach 99%. Aeration in the dual-chamber cell cathode can blow out more than 99% of the ammonia nitrogen that is converted from NO3−-N. More than 95% of the TN in a 100 ml, 700 mg/L NO3−-N solution was removed within 6 h. CuNi/M/GF showed excellent corrosion resistance and stability in the cyclic tests. CuNi–1.3/M/GF is more resistant to corrosion in the DCC than in the SCC which may be attributed to contact of the cathode with the oxidizing substance in the SCC. The application of CuNi/M/GF in practical wastewater that contained a high concentration of NaCl and nitrate-N shows that the electrode has considerable application prospects in TN removal.
Graphical abstract







Similar content being viewed by others
References
Martínez J, Ortiz A, Ortiz I (2017) State-of-the-art and perspectives of the catalytic and electrocatalytic reduction of aqueous nitrates. Appl Catal B 207:42–59. https://doi.org/10.1016/j.apcatb.2017.02.016
Shrimali M, Singh KP (2001) New methods of nitrate removal from water. Environ Pollut 112:351–359
Bilanovic D, Battistoni P, Cecchi F, Pavan P, Mata-Alvarez J (1999) Denitrification under high nitrate concentration and alternating anoxic conditions. Water Res 33:3311–3320
Vitousek PM, Aber JD, Howarth RW et al (1997) Technical report: human alteration of the global nitrogen cycle: sources and consequences. Ecol Appl 7:737–750. https://doi.org/10.2307/2269431
Fanning JC (2000) The chemical reduction of nitrate in aqueous solution. Coord Chem Rev 199:159–179
Risica S (2011) Guidelines for drinking-water quality, 4th edn. WHO, Geneva
Rivett MO, Buss SR, Morgan P, Smith JW, Bemment CD (2008) Nitrate attenuation in groundwater: a review of biogeochemical controlling processes. Water Res 42:4215–4232. https://doi.org/10.1016/j.watres.2008.07.020
USEPA (2017) Ground water and drinking water table of regulated drinking water contaminants
Kalaruban M, Loganathan P, Kandasamy J, Vigneswaran S (2018) Submerged membrane adsorption hybrid system using four adsorbents to remove nitrate from water. Environ Sci Pollut Res 25:20328–20335. https://doi.org/10.1007/s11356-017-8905-9
Afonso MD, Jaber JO, Mohsen MS (2004) Brackish groundwater treatment by reverse osmosis in Jordan. Desalination 164:157–171
Kim YM, Kim SJ, Kim YS, Lee S, Kim IS, Kim JH (2009) Overview of systems engineering approaches for a large-scale seawater desalination plant with a reverse osmosis network. Desalination 238:312–332
Chen Y, Peng C, Wang J, Liu Y, Zhang L, Peng Y (2011) Effect of nitrate recycling ratio on simultaneous biological nutrient removal in a novel anaerobic/anoxic/oxic (A2/O)-biological aerated filter (BAF) system. Bioresour Technol 102:5722–5727
Ding J, Li W, Zhao Q-L, Wang K, Zheng Z, Gao Y-Z (2015) Electroreduction of nitrate in water: role of cathode and cell configuration. Chem Eng J 271:252. https://doi.org/10.1016/j.cej.2015.03.001
Wang Q, Zhao X, Zhang J, Zhang X (2015) Investigation of nitrate reduction on polycrystalline Pt nanoparticles with controlled crystal plane. J Electroanal Chem 755:210–214. https://doi.org/10.1016/j.jelechem.2015.08.005
Mattarozzi L, Cattarin S, Comisso N et al (2013) Electrochemical reduction of nitrate and nitrite in alkaline media at CuNi alloy electrodes. Electrochim Acta 89:488–496. https://doi.org/10.1016/j.electacta.2012.11.074
Comisso N, Cattarin S, Guerriero P et al (2016) Study of Cu, Cu-Ni and Rh-modified Cu porous layers as electrode materials for the electroanalysis of nitrate and nitrite ions. J Solid State Electrochem 20:1139–1148
Birdja YY, Yang J, Koper MTM (2014) Electrocatalytic reduction of nitrate on tin-modified palladium electrodes. Electrochim Acta 140:518–524. https://doi.org/10.1016/j.electacta.2014.06.011
Katsuaki S (2004) High electrocatalytic performance of Pd/Sn/Au electrodes for nitrate reduction. J Electroanal Chem 572:93–99
Garcia-Segura S, Lanzarini-Lopes M, Hristovski K, Westerhoff P (2018) Electrocatalytic reduction of nitrate: fundamentals to full-scale water treatment applications. Appl Catal B 236:546–568. https://doi.org/10.1016/j.apcatb.2018.05.041
de Groot MT, Mtm K (2004) The influence of nitrate concentration and acidity on the electrocatalytic reduction of nitrate on platinum. J Electroanal Chem 562:81–94
Jha K, Weidner JW (1999) Evaluation of porous cathodes for the electrochemical reduction of nitrates and nitrites in alkaline waste streams. J Appl Electrochem 29:1305–1315
Radjenovic J, Sedlak DL (2015) Challenges and opportunities for electrochemical processes as next-generation technologies for the treatment of contaminated water. Environ Sci Technol 49:11292–11302. https://doi.org/10.1021/acs.est.5b02414
Mattarozzi L, Cattarin S, Comisso N et al (2014) Hydrogen evolution assisted electrodeposition of porous Cu-Ni alloy electrodes and their use for nitrate reduction in alkali. Electrochim Acta 140:337–344
Wang Q, Yue K, Zhang X, Wang L, Guiver MD, Zhang J (2017) Carbon fiber paper supported nano-Pt electrode with high electrocatalytic activity for concentrated nitric acid reduction. J Electroanal Chem 794:43–48. https://doi.org/10.1016/j.jelechem.2017.03.043
Zhu W, Zhang X, Yin Y, Qin Y, Zhang J, Wang Q (2018) In-situ electrochemical activation of carbon fiber paper for the highly efficient electroreduction of concentrated nitric acid. Electrochim Acta 291:328–334. https://doi.org/10.1016/j.electacta.2018.08.127
Zhang X, Zhang S, Zhu W et al (2020) Synergetic electrochemical HNO3 reduction on the activated-CFP supported nano-Pt electrodes. J Electroanal Chem 869:114182. https://doi.org/10.1016/j.jelechem.2020.114182
Zhang Y, Zhao Y, Chen Z, Wang L, Wu P, Wang F (2018) Electrochemical reduction of nitrate via Cu/Ni composite cathode paired with Ir-Ru/Ti anode: high efficiency and N2 selectivity. Electrochim Acta 291:151–160. https://doi.org/10.1016/j.electacta.2018.08.154
Wu Y, Gan L, Zhang S et al (2018) Carbon-nanotube-doped Pd-Ni bimetallic three-dimensional electrode for electrocatalytic hydrodechlorination of 4-chlorophenol: enhanced activity and stability. J Hazard Mater 356:17–25. https://doi.org/10.1016/j.jhazmat.2018.05.034
Su L, Li K, Zhang H et al (2017) Electrochemical nitrate reduction by using a novel Co3O4/Ti cathode. Water Res 120:1–11. https://doi.org/10.1016/j.watres.2017.04.069
Chang J-K, Hsu S-H, Sun IW, Tsai W-T (2008) Formation of nanoporous nickel by selective anodic etching of the nobler copper component from electrodeposited nickel−copper alloys. J Phys Chem C 112:1371–1376. https://doi.org/10.1021/jp0772474
Liu Z, Xia G, Zhu F et al (2008) Exploiting finite size effects in a novel core/shell microstructure. J Appl Phys 103:064313. https://doi.org/10.1063/1.2844286
Reyter D, Bélanger D, Roué L (2011) Optimization of the cathode material for nitrate removal by a paired electrolysis process. J Hazard Mater 192:507–513. https://doi.org/10.1016/j.jhazmat.2011.05.054
Durivault L, Brylev O et al (2007) Cu-Ni materials prepared by mechanical milling: their properties and electrocatalytic activity towards nitrate reduction in alkaline medium. J Alloys Compd 432:323–332
Hristovski KD, Markovski J (2017) Engineering metal (hydr) oxide sorbents for removal of arsenate and similar weak-acid oxyanion contaminants: a critical review with emphasis on factors governing sorption processes. Sci Total Environ 598:258–271. https://doi.org/10.1016/j.scitotenv.2017.04.108
Cook AR, Dimitrijevic N, Dreyfus BW, Meisel D, Curtiss LA, Camaioni DM (2001) Reducing radicals in nitrate solutions. The NO32-system revisited. J Phys Chem A 105:3658–3666. https://doi.org/10.1021/jp0038052
Dima GE, Beltramo GL, Koper MTM (2005) Nitrate reduction on single-crystal platinum electrodes. Electrochim Acta 50:4318–4326. https://doi.org/10.1016/j.electacta.2005.02.093
de Vooys ACA, Beltramo GL, van Riet B, van Veen JAR, Koper MTM (2004) Mechanisms of electrochemical reduction and oxidation of nitric oxide. Electrochim Acta 49:1307–1314. https://doi.org/10.1016/j.electacta.2003.07.020
Horányi G, Rizmayer EM (1982) Role of adsorption phenomena in the electrocatalytic reduction of nitric acid at a platinized platinum electrode. J Electroanal Chem Interfacial Electrochem 140:347–366. https://doi.org/10.1016/0022-0728(82)85178-4
Katsounaros I, Kyriacou G (2008) Influence of nitrate concentration on its electrochemical reduction on tin cathode: identification of reaction intermediates. Electrochim Acta 53:5477–5484. https://doi.org/10.1016/j.electacta.2008.03.018
Katsounaros I, Kyriacou G (2007) Influence of the concentration and the nature of the supporting electrolyte on the electrochemical reduction of nitrate on tin cathode. Electrochim Acta 52:6412–6420. https://doi.org/10.1016/j.electacta.2007.04.050
Li M, Feng C, Zhang Z, Sugiura N (2009) Efficient electrochemical reduction of nitrate to nitrogen using Ti/IrO2–Pt anode and different cathodes. Electrochim Acta 54:4600–4606. https://doi.org/10.1016/j.electacta.2009.03.064
Li M, Feng C, Zhang Z et al (2009) Simultaneous reduction of nitrate and oxidation of by-products using electrochemical method. J Hazard Mater 171:724–730. https://doi.org/10.1016/j.jhazmat.2009.06.066
Couto AB, Oishi SS, Ferreira NG (2016) Enhancement of nitrate electroreduction using BDD anode and metal modified carbon fiber cathode. J Ind Eng Chem 39:210–217. https://doi.org/10.1016/j.jiec.2016.05.028
Karri RR, Sahu JN, Chimmiri V (2018) Critical review of abatement of ammonia from wastewater. J Mol Liq 261:21–31. https://doi.org/10.1016/j.molliq.2018.03.120
Hl Li DH, Robertson JQC, Hobbs DT (1988) Electrochemical reduction of nitrate and nitrite in concentrated sodium hydroxide at platinum and nickel electrodes. J Electrochem Soc 135:1154. https://doi.org/10.1149/1.2095900
Paidar M, Roušar I, Bouzek K (1999) Electrochemical removal of nitrate ions in waste solutions after regeneration of ion exchange columns. J Appl Electrochem 29:611–617. https://doi.org/10.1023/A:1026423218899
Li W, Xiao C, Zhao Y, Zhao Q, Fan R, Xue J (2016) Electrochemical reduction of high-concentrated nitrate using Ti/TiO2 nanotube array anode and Fe cathode in dual-chamber cell. Catal Lett 146:2585–2595. https://doi.org/10.1007/s10562-016-1894-3
Acknowledgements
We gratefully acknowledge generous support provided by the National Natural Science Foundation (No. 51778281) and Jiangsu Natural Science Fund (Nos. BK20171342 and BK20161493) P.R. China, and this study is supported by the Fundamental Research Funds for the Central Universities (No. 20620140479), the Nanjing Normal University Research Funding (184080H202B146), the State Key Program of National Natural Science of China (No. 51438008), State Key Laboratory of Pollution Control and Resource Reuse Open Fund (PCRRF18018, PCRRF19032), and National Key R&D Program of China (No. 2016YFE0112300).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Christopher Blanford.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Lu, C., Lu, X., Yang, K. et al. Cu, Ni and multi-walled carbon-nanotube-modified graphite felt electrode for nitrate electroreduction in water. J Mater Sci 56, 7357–7371 (2021). https://doi.org/10.1007/s10853-020-05764-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-020-05764-3