Abstract
Cyclic N-methylol compounds such as 1,3-dimethylol-4,5-dihydroxyethyleneurea (DMDHEU) have been used to modify wood and prevent negative effects related to the uptake of moisture. However, the changes in the sorption behavior of wood by treatments with DMDHEU and its derivatives are not fully understood. In the present study, wood blocks were treated with DMDHEU, ether-modified DMDHEU and diethyleneglycolated DMDHEU in order to study the factors that control the changes in moisture uptake in the hygroscopic range (0–95% RH). Dimensional changes of wood blocks during water soaking cycles suggested that the treatments caused a permanent cell wall bulking, whereas the swelling restraint by cross-linking of adjacent cell wall polymers was not permanent. However, the changes in water vapor sorption were not only a result of the cell wall bulking effect that reduced the space in the cell wall to accommodate water. The N-methylol compounds within the wood also provided additional sorption sites, but there was no correlation between absorbed water and accessible OH groups. It was speculated that the co-condensation of the N-methylol compounds with wood polymers had a significant effect on the sorption of the treated wood. At elevated RH, pure resins that were formed by self-condensation took up large quantities of moisture. However, when the N-methylol compounds were heat-cured within the hierarchical structure of wood, the moisture uptake of the treated wood at elevated RH was even lower compared to unmodified wood. Furthermore, the covalent bond formation between wood and resin prolonged the attainment of an equilibrium moisture content.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cyclic N-methylol compounds such as 1,3-dimethylol-4,5-dihydroxyethyleneurea (DMDHEU) can be used to modify wood and improve its service life in exterior applications [1, 2]. Compared to untreated wood, DMDHEU-treated wood has an improved dimensional stability and decay resistance [3, 4]. The treatment with N-methylol compounds also affects the water vapor sorption of wood in the hygroscopic range [5, 6]. However, the underlying modes of action are not fully understood, because the water sorption of the treated wood is influenced by a number of factors, such as the location of the compounds within the hierarchical structure of wood or the additional moisture uptake of the resin.
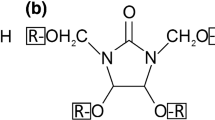
Originally, cyclic N-methylol compounds like DMDHEU were developed and used for finishing cellulose-based fabrics [7], but can also be applied for the impregnation modification of wood [1]. Water-soluble DMDHEU monomers are synthesized from urea, glyoxal and formaldehyde, with two hydroxymethyl groups (–CH2–OH) being the reactive functional groups in this molecule. They can be ether-modified (mDMDHEU) by reaction with methanol (CH3) and further modified using diethylene glycol (C4H10O3, DEG). Compared to DMDHEU, mDMDHEU causes lower formaldehyde emissions, but also results in a lower DMDHEU reactivity [8, 9]. During a heat cure at elevated temperature, the formation of ether bonds by reaction of the hydroxymethyl groups with hydroxyl (OH) groups of cellulose (co-condensation) or with OH groups of adjacent DMDHEU monomers (self-condensation) is the reaction mechanisms of DMDHEU in cellulose-based fabrics [7].
Dissolved in water, DMDHEU molecules are small enough to enter the nanopores of the water-swollen wood cell wall during a vacuum pressure impregnation step [3, 4]. A subsequent heat cure at elevated temperatures (> 100 °C) causes the fixation of the DMDHEU molecules within the wood structure. The expected reactions are similar to cellulose-based fabrics; thus, the formation of large macromolecules via self-condensation and the reaction between wood and resin via co-condensation should fixate the N-methylol compounds in the wood structure permanently. The cured resin fills the space between cell wall matrix polymers to keep the treated wood in a permanently swollen state [3]. This effect is denoted as ‘cell wall bulking’ and commonly measured on a macroscopic level by the increase in dry dimensions of the modified wood blocks.
The presence of DMDHEU in the wood cell wall decreases moisture-induced dimensional changes and alters the void size, cell wall pore size and accessibility of hydroxyl (OH) groups [5, 10]. Cell wall bulking by chemical agents may reduce the uptake of liquid water in the cell wall [11] and MC of wood independent of the RH level in the hygroscopic [12] and over-hygroscopic range [13]. Although cell wall bulking may block some of the accessible OH groups via steric hindrance [14], the main effect is the reduced spatial availability in the treated cell wall for water [15]. In addition to a cell wall bulking effect, [16] found indications for a cross-linking effect caused by treatments with DMDHEU. They recorded a significant reduction in the water-saturated dimensions of treated wood blocks, which suggested that the DMDHEU formed cross-links between wood polymers to restrict the water-induced swelling of the cell wall. Based on treatments with paraformaldehyde, the swelling restriction by the cross-linking effect is believed to reduce the wood moisture content particularly at elevated relative humidities [12]. However, more recent studies found no evidence for a cross-linking effect in wood treated with N-methylol compounds [17].
The moisture exclusion of the treated wood by the cell wall bulking and/or cross-linking effect may be counterbalanced by the ability of resins to take up moisture [18]. Monomers of glyoxal resins like DMDHEU contain free OH groups which constitute 38% of the molecular weight [7]. The provision of additional water sorption sites by the treatment of wood with DMDHEU has already been shown by [6]. The contribution of the resins may especially be important in regard to potential resin deposits in the cell lumen, which would provide additional sorption sites without causing a moisture exclusion effect in the cell wall. However, to our knowledge, water sorption isotherms of DMDHEU resins have not been studied systematically, but this may help in understanding the interaction between wood and resin with regard to the sorption behavior of the formed composite material.
The present study investigated how treatments with DMDHEU and its derivatives affect the water vapor sorption behavior of wood in the hygroscopic range (0–95% RH). Potential moisture exclusion effects by cell wall bulking or swelling restraints were investigated on treated wood blocks. Changes in accessible OH group content of the treated wood, either by reaction of OH groups in wood with the N-methylol compounds or by the additional OH groups in the resins, were quantified by hydroge N-deuterium exchange. Furthermore, the sorption behavior of the pure resins was studied after heat-curing of the N-methylol compounds without the presence of wood.
Materials and methods
Material and treatment process
Scots pine sapwood (Pinus sylvestris L.) with dimensions of 25 × 25 × 10 mm3 (radial × tangential × longitudinal) and an average density of 0.48 g cm−3 was used for the experiments. Prior to the treatment, all specimens were oven-dried at 103 (± 2) °C for 48 h to determine the initial dry mass and dimensions. The oven-dried specimens were treated with aqueous solutions of 1,3-dimethylol-4,5-dihydroxyethyleneurea (DMDHEU), methylated DMDHEU (mDMDHEU) and diethyleneglycolated DMDHEU (mDMDHEU + DEG, Fig. 1). The solutions were diluted with deionized water to solid contents of 10 or 20%. Magnesium nitrate (Mg(NO3)2) was added as catalyst, in a concentration of 1.16% related to the added resin solid content. The specimens were impregnated with the treatment solutions by applying vacuum (5 kPa) for 1 h, followed by an over-pressure phase at 1200 kPa for another 1 h. The fully impregnated specimens were removed from the treatment solution and stored at room climate for 72 h before they were cured in an oven at 120 °C for 48 h. In addition to wood specimens, approximately 15 g of the different stock solutions were also heat-cured using the same temperature and duration. The cured wood specimens were cooled down in a desiccator over silica gel before the dry mass and dimensions were recorded. All specimens, except those tested in water-soaking cycles, underwent a cold-water leaching with deionized water according to [19]. Subsequently, they were dried at ambient conditions for ca. 24 h and in an oven at 103 °C for another 24 h to determine the final dry mass and dimensions. Weight percent gain (WPG, in %) and cell wall bulking (CWB, in %) were calculated based on the initial and final dry mass and dimensions using Eqs. 1 and 2:
where minitial and mfinal are the initial dry mass and the final dry mass after treatment and water leaching, respectively, while Ainitial and Afinal are the corresponding initial and final dry cross-sectional areas (radial × tangential).
Fourier-transform infrared spectroscopy
Fourier-transform infrared (FT-IR) spectra were collected using a Bio-Rad FTS-6000 spectrometer (Cambridge, USA) with a MTEC 300 photoacoustic detector (Ames, USA) using a 10 kHz mirror velocity and a resolution of 8 cm−1. Before measuring samples, the background spectrum was measured with standard carbon black in the detector. To measure wood samples, small sections of either pure latewood or pure earlywood were cut from the water-leached and oven-dried wood blocks with a razor blade. Additional measurements were done on small pieces of cured resin. The samples were put into a detection cell, which was placed into the detector. The cell was flushed with helium for 5 min before it was sealed to record the spectrum. For each measurement, 400 scans were collected and processed with the WIN-IR Pro 3.4 software (Digilab, Randolph, MA, USA). Spectra of cured resin were normalized by the area below each spectrum in the wave number range between 750 and 3700 cm−1 and average spectra of two measurements per resin are displayed. The wood spectra were normalized to the absorbance at ca. 1508 cm−1 and average spectra of five measurements per sample group are displayed. From the baseline-corrected and normalized wood spectra, the band at ca. 1720 cm−1 in the treated wood was quantified by integration between 1800 and 1628 cm−1 with the absorbance being set to zero at these thresholds.
Water-soaking cycles
A separate set of specimens was exposed to water-soaking cycles to record changes in mass and dimensions. This test was conducted with ten replicates per resin type (DMDHEU, mDMDHEU or mDMDHEU + DEG), using only specimens that were treated using impregnation solutions with 20% solid content. The specimens were subjected to five water-soaking cycles that included a water saturation step and a drying step, as described by [20]. Water saturation was achieved by vacuum impregnation with deionized water for 30 min at 5 kPa, which was followed by soaking in water for 24 h. Re-drying was done by storing the wet samples at ambient conditions for 24 h, followed by oven-drying at 103 °C for another 24 h. Untreated Scots pine sapwood specimens served as reference specimens. The specimens’ mass and dimensions (radial × tangential) during the water-soaking cycles are given as relative values by relating each mass and dimension to the initial dry mass and initial dry dimension of the specimens before the modification, as described by [21]. Furthermore, relative swelling was calculated by subtracting the relative dry dimensions from the corresponding wet dimensions of the same cycle.
Hydrogen -deuterium exchange
Solid wood blocks were milled in a cutting mill (RETSCH SM 2000, Retsch GmbH, Haan, Germany) to pass through a 2 mm mesh screen. Hydrogen–deuterium exchange (HDX) was performed in a dynamic vapor sorption (DVS) apparatus (DVS ET, Surface measurement Systems, London, UK) as described previously [18]. In short, approximately 20 mg of wood particles were first dried using the pre-heater at 60 °C for 6 h under dry nitrogen flow (0% RH) followed by a temperature stabilization period to 25 °C for 2 h. The particles were then exposed to deuterium oxide (D2O) vapor at a target RH of 95% for 12 h. Finally, the dry mass was determined again as described above. All measurements were done in triplicate. The amount of exchanged H and the amount of absorbed D2O (both in mmol g−1) was calculated as described by [18] using either the dry specimen mass or the dry wood mass (excluding the mass of the added resin) as the reference mass for the calculations.
Sorption isotherms
Sorption isotherms were recorded in a DVS apparatus (DVS Advantage, Surface Measurement Systems, London, UK). Besides the wood particles that were also used for HDX measurements, the measurements were also performed on small pieces that were cut from the cured resins. Approximately 20 mg of wood particles or resin pieces were placed on the sample holder of the DVS, and sorption isotherms were recorded at a constant temperature of 25 °C and a nitrogen flow of 200 sccm (standard cubic centimeter at 0 °C, 100 kPa per minute according to IUPAC). The samples were first dried at 0% RH until the mass change of the specimen per minute (dm/dt) was < 0.001% min−1 over a period of 10 min and then exposed to a RH of 95% for 720 min. This was done to limit the potential influence of the previous sorption history on the sorption isotherms, which has been observed when comparing multiple sorption cycles [22,23,24]. Afterwards, the samples were dried again at 0% RH before the RH was increased stepwise in the following sequence: 5, 15, 25, 35, 45, 55, 65, 75, 85, and 95% RH (absorption curve), which was followed by a decrease to 0% RH in the reverse order (scanning desorption curve). A 10 min window was used to calculate the dm/dt, and each RH was maintained until the dm/dt was < 0.001% min−1 over a period of 10 min. The moisture content (MC, in %) was calculated by relating the mass of water to the dry sample mass using the sample mass at the end of each RH step. This MC was corrected for the mass added by the modification agent as described by [25]. This corrected MC is denoted MCR and related the mass of absorbed water to the dry wood mass. Furthermore, the MCR ratio was calculated by relating the MCR of the modified sample to the corresponding reference MC at each RH step.
Water vapor sorption over saturated salt solutions
Recent studies showed short holding times during dynamic vapor sorption (DVS) measurements misrepresenting the equilibrium moisture content of a certain wood-based material [26, 27]. Hence, additional water vapor tests were performed over long periods using saturated salt solutions. Wood specimens of 25 × 25 × 10 (ax.) mm3 were split into smaller blocks of approx. 12.5 × 12.5 × 10 mm3 (radial × tangential × longitudinal). The blocks were oven-dried at 103 °C for 48 h, and the dry mass was measured after cooling in a desiccator over silica gel. The dried blocks were placed on perforated, stainless steel plates over saturated, aqueous salt solutions in a sealed plastic container. Sealed containers were stored in a temperature-controlled room at approx. 20 °C. According to [28], saturated solutions of sodium chloride (NaCl) and potassium sulfate (K2SO4) were used to achieve RH levels of 75% and 97%. The mass of each wood block was recorded weekly on two consecutive days, using a laboratory balance with an accuracy of 10–1 mg. Saturated salt solutions were stirred weekly with a whisk at the time of weighing the specimens and opening the containers. Conditioning was started with NaCl and the salt solution exchanged to K2SO4 when the change in MC between two consecutive days was less than 0.01% day−1. MCR and MCR ratios were calculated as described for the DVS analysis.
Results and discussion
FT-IR spectroscopy
The presence of the different resins within the early- and the latewood of the treated blocks was confirmed by FT-IR spectroscopic measurements (Fig. 1d). There was a lot of overlap in IR absorbance of wood and the cured resins, but little to no overlap was found for the lignin-related band at ca 1508 cm−1 in untreated wood that was nearly absent in the FT-IR spectra of the cured resins. One distinct difference between wood and cured resins was the strong absorbance of the resins at ca. 1724 cm−1. Unreacted, monomeric DMDHEU shows a band at ca. 1695 cm−1, and this band shifts to larger wave numbers (to 1724 cm−1 in the present study) upon polycondensation that results in the formation of ether bonds [29]. In mDMDHEU, the reaction of the DMDHEU with methanol may also contribute to this band; thus, a larger absorbance at ca. 1724 cm−1 was found in the FT-IR spectra of resins formed from mDMDHEU and mDMDHEU + DEG. The FT-IR spectra also confirmed the presence of OH groups in the cured resins by the broad OH stretching band around 3340 cm−1. Furthermore, all cured resins showed FT-IR bands at ca. 2943, 1466 and 1246 cm−1, which could be assigned to the C–H stretching vibration of the methylene groups, –CH2 stretching vibration and –CHOH stretching vibration, respectively [30]. In case of DMDHEU, the –CH2 stretching band was less intense and had a maximum at slightly higher wave numbers (ca. 1477 cm−1). In methylated DMDHEU, an additional C–H stretching band was formed at 2885 cm−1. Furthermore, a large band structure was found between 1150 and 960 cm−1, which was presumably related to C–N and C–O groups in the cured resins [3]. For methylated and diethyleneglycolated DMDHEU (mDMDHEU and mDMDHEU + DEG), the band maximum was at ca. 1095 cm−1, whereas it was ca. 1057 cm−1 for DMDHEU.
The FT-IR spectra of the treated wood specimens are shown in Fig. 2a and b. Their absorbance was normalized to the band at ca. 1597 cm−1, which can be assigned to the aromatic skeletal vibration in lignin and C=O stretching vibration in wood [31, 32]. The treatment increased the absorbance of the bands at ca. 1458 and 1242 cm−1 compared to untreated wood, which can be explained by the absorbance of the resins at similar wave numbers. However, the most obvious change in the FT-IR spectrum of wood by the treatment with the resins was the intense band at ca. 1720 cm−1, which overlaid the carbonyl band in native wood (ca. 1736 cm−1) and originated from the ether bonds formed in DMDHEU and ether-modified DMDHEU. Xie et al. [29] reported that the intensity of this band increased when the solid content of the impregnation solution was increased between 10 and 50%. However, such an increase was only found for treatments with mDMDHEU and mDMDHEU + DEG, while the absorbance at 1720 cm−1 was nearly identical after treatments with a 10 and 20% solution of DMDHEU.
Results of the FTIR spectroscopic measurements of treated wood: a average spectra normalized to the band at ca. 1508 cm−1, b close up view of the wave number region 1850–1200 cm−1 and c band area (% of reference) within the 1800–1628 cm−1 region in dependence on the chemical agent and the solid content applied. Each column represents an average of five measurements, and the error bars show the standard deviation
The intensity of the carbonyl band was also quantified by integration from 1800 to 1628 cm−1 (Fig. 2c). Besides effects of the solid content and the chemical agent used, the band area also differed between early- and latewood. Most probably, the larger cell lumens in earlywood enabled a higher uptake of the impregnation solution in comparison with latewood. It remained unclear, however, whether the additional resin in the earlywood was located in the cell lumen or if this resulted in a higher diffusion of resin into the cell wall. The results of the band area also confirmed the nearly identical intensity of the carbonyl band for wood treated with a 10 or 20% solution of DMDHEU. Besides the formation of ether bonds by self-condensation, the etherification of wood OH groups may have further contributed to the band at 1720 cm−1. Therefore, the increase in band area shown in Fig. 2c may not be an accurate measure for the number of DMDHEU units in the wood. This is also in line with the higher increase in band area after treatments with a 20% solution of mDMDHEU and mDMDHEU + DEG compared to DMDHEU. The number of DMDHEU units was slightly higher in the DMDHEU solution, but the ether bonds that were already present in the mDMDHEU due to the reaction with methanol presumably resulted in a stronger increase in the band at ca. 1720 cm−1 after treatments with methylated or diethyleneglycolated mDMDHEU.
Changes in mass and dimensions by the treatment with N-methylol compounds
Treatments with DMDHEU, methylated DMDHEU (mDMDHEU) and diethyleneglycolated DMDHEU (mDMDHEU + DEG) caused an increase in dry mass and dry dimensions of the wood blocks, which are illustrated as weight percent gain (WPG) and cell wall bulking (CWB), respectively (Table 1). The increase in WPG and CWB was proportional to the solid content of the impregnation solution, which corresponded to earlier studies with N-methylol compounds [3, 33]. After a cold-water leaching, only small losses in WPG and CWB were recorded, which indicated a high fixation of the N-methylol compounds in the wood. Negative WPG and CWB were measured for the water-leached reference samples; hence, water-soluble extractives in the untreated wood contributed to the loss in dry mass and dimensions during the cold-water leaching.
The WPG was nearly identical for the different glyoxal resins. It should be noted, however, that the number of DMDHEU units within the treated wood most likely differed, because methylol groups and DEG contributed to the mass increase during treatments with mDMDHEU and mDMDHEU + DEG. In contrast to the WPG, the CWB increased when mDMDHEU and mDMDHEU + DEG were used instead of DMDHEU. For impregnation solutions with a solid content of 20%, this difference was still noticeable after the cold-water leaching, and a CWB of 2.4, 3.3 and 3.8% was recorded for DMDHEU, mDMDHEU and mDMDHEU + DEG, respectively. This difference in CWB corresponded to earlier studies on DMDHEU and ether-modified DMDHEU [17]. It indicated that a higher volume of water-accessible cell wall pore space was occupied after treatments with mDMDHEU and mDMDHEU + DEG instead of DMDHEU. Besides the pore size of the swollen cell wall, the molar mass and the polarity of the molecules are crucial parameters for a cell wall diffusion [34]. Therefore, a higher CWB of wood after treatments with mDMDHEU instead of DMDHEU can be assigned to the increased molar mass and higher polarity [35] that promote the cell wall diffusion of the resin.
Changes in mass and dimensions by water-soaking cycles
Changes in mass and dimensions were further analyzed during repeated cycles of water saturation and re-drying using a separate set of specimens that were treated using impregnation solutions with a 20% solid content (Fig. 3). The treatment with the cyclic N-methylol compounds aims to reduce moisture-induced dimensional changes. This reduction results either from a permanent cell wall bulking due to molecule deposition inside the cell wall [36], from the cross-linking of adjacent cellulose fibrils that restricts the cell wall swelling [12, 37] or from a combination of both [16]. As illustrated in Fig. 3a, a cell wall bulking effect would result in an increase in dry dimensions, while a swelling restraint by cross-linking of cell wall polymers would cause the reduction in water-saturated dimensions; hence, these two modes of action can be differentiated during the water-soaking cycles.
Changes in sample mass and dimensions during water-soaking cycles: Schematic illustration of the expected dimensional changes caused by cell wall bulking or a swelling restraint a, as well as changes in relative dry mass b, relative dry dimensions c, relative water-saturated dimensions d and relative swelling directly after the treatment and in the course of five water-soaking cycles. Each data point represents an average of ten samples, and the error bars show the standard deviation. Note the breaks in the y-axes in b and c
After the modification with a 20% solid content, the presence of DMDHEU and ether-modified DMDHEU in wood increased the dry mass (Fig. 3b) and dry dimensions of the wood blocks (Fig. 3c). The dry mass experienced a slight decrease after five wetting–drying cycles (Fig. 3b), while the dry dimensions remained almost constant over all cycles (Fig. 3c). Therefore, the loss in dry mass was presumably caused by the leaching of unreacted chemicals from the cell lumen. Untreated wood blocks experienced continuous losses in dry mass and dry dimensions due to the removal of water-soluble extractives from the cell wall.
The water-saturated dimensions during the first water-soaking cycle were smaller when compared to the dimensions after impregnation, and this was most noticeable after a treatment with mDMDHEU (Fig. 3d). This reduction in water-saturated dimensions indicated a cross-linking effect that restrained the swelling of the modified wood blocks. However, this effect disappeared gradually in the course of five drying-wetting cycles, which resulted in an increase in relative swelling with increasing number of water-soaking cycles (Fig. 3e). After five water-soaking cycles, the water-saturated dimensions of the wood blocks that were treated with DMDHEU and mDMDHEU + DEG exceeded the dimensions of untreated wood, and a small reduction in water-saturated dimensions was only observed for wood treated with mDMDHEU. A similar effect of a loss in dimensional stability during water-soaking cycles had been observed in thermally modified wood [38,39,40]. The exact cause for the increase in water-saturated dimensions of the treated wood is unclear. However, it may be speculated that the stresses during the repeated wetting and re-drying caused a realignment of the polymers within the cell wall until the wood could swell to its initial water-saturated dimensions.
The present results contradict with the results of [16], who found a permanent decrease in water-saturated dimensions after treatments of Scots pine sapwood with DMDHEU or ether-modified DMDHEU, followed by the Soxhlet extraction with hot water. However, later studies disconfirmed the reduction in water-saturation dimensions by treatments with DMDHEU [4, 41]. It is thus concluded that the reduction in the volumetric margin between the oven-dry and water-saturated state of the wood after treatment with cyclic N-methylol compounds relies primarily on a cell wall bulking effect, with little or no contribution from a swelling restraint caused by cross-linking of adjacent cell wall polymers.
Accessible sorption sites in treated wood
Accessible sorption sites were quantified by the HDX approach, which has previously been applied to thermally modified wood [42, 43] and wood that was treated using carboxylic acid anhydrides [14, 44], furfuryl alcohol [45] or melamine formaldehyde [18]. The results were illustrated by relating the mass of exchanged H or absorbed D2O either to the dry sample mass or the dry wood mass (Fig. 4). Using the dry samples mass as reference mass gives the correct sorption site density of the treated wood as a composite of wood and resin. However, the increase in dry sample mass by the treatment complicates the comparison of the differently treated wood blocks. On the other hand, using the dry wood mass as reference mass overestimates the exchanged H and the amount of absorbed D2O of the treated wood, but the interpretation of the results is easier when only the numerator is changed while the denominator (reference mass) remains constant.
Results of the hydrogen–deuterium exchange measurements: Exchanged hydrogen (a) and absorbed D2O (b, both in mmol g−1) in dependence on the solid content of the respective impregnation solution. The calculations are based either on the dry sample mass (filled bars) or the dry wood mass (patterned columns) as reference mass. The correlation between absorbed D2O and exchanged H (both based on dry wood mass) are shown in (c). Note the breaks in the y-axes in (a) and (b)
The amount of exchanged H increased with increasing solid content of the impregnation solution when related to the dry wood mass, while the opposite was found when related to the dry sample mass (Fig. 4a). This is because the cured resins contained sorption sites that contributed to the amount of exchanged H, but the concentration in the resins was lower than the concentration of sorption sites in untreated wood. At the same solid content, the concentration of sorption sites increased in the order of mDMDHEU + DEG, mDMDHEU and DMDHEU, which correlated with the increase in CWB in the reverse order. With a higher CWB, the modification agents may be more effective in blocking of accessible OH groups by steric hindrance, as suggested by [14]. Further on, when applying DMDHEU, methylated or diethyleneglycolated DMDHEU at constant solid content to wood, the number of DMDHEU units with exchangeable H decreases in the order of DMDHEU, mDMDHEU and mDMDHEU + DEG [46, 47]. However, DEG is split off during the cure of diethyleneglycolated DMDHEU (mDMDHEU + DEG) so that DEG remains in the wood structure and is expected to provide additional sorption sites for water [47].
The concentration of absorbed D2O molecules at 95% target RH was also derived from the HDX measurements (Fig. 4b). There were some similarities with the concentration of sorption sites, in particular the amount of absorbed D2O increased with the solid content of the impregnation solution and the amount of absorbed D2O was larger for treatments with DMDHEU than with methylated or diethyleneglycolated DMDHEU. However, the amount of absorbed D2O in the treated wood decreased or remained almost constant compared to an untreated control sample, while the amount of exchanged H increased when the wood mass was used as reference mass. Furthermore, no correlation between adsorbed D2O and exchanged H was found (Fig. 4c). This finding was in line with HDX experiments on cotton cellulose that was treated with different N-methylol compounds [48]. Furthermore, a number of recent wood-related studies showed that changes in hydroxyl accessibility by thermal, chemical or resin treatments are not the only factor that determines moisture absorption of wood [18, 42, 49]. Thybring et al. [15] showed that the dominant factor in reducing moisture uptake in chemically modified wood is the reduced spatial availability for water inside the cell walls. Such reduced spatial availability has also been shown to counterbalance the additional moisture uptake by melamine formaldehyde resin in resin-treated wood [18]. A similar effect presumably applied for wood treated with N-methylol compounds, too. However, the outcome was presumably more complex, because the N-methylol compounds provided additional sorption sites, while also reacting with accessible OH groups in the wood.
Sorption behavior of cured resins
Both types of cured resins—DMDHEU and ether-modified DMDHEU—contain polar groups that may interact with water molecules [47], which was also shown by the FT-IR spectra and the HDX experiments. Therefore, sorption isotherms of cured DMDHEU and ether-modified DMDHEU were measured on small pieces of cured resin. Absorption and (scanning) desorption isotherms of the cured resins resembled an IUPAC type III isotherm (Fig. 5). Only small moisture changes were recorded up to a RH of ca. 50%, whereas a steep increase in MC was measured when the RH was further increased. This RH-dependent course of the MC was similar to the sorption isotherm of incompletely cured melamine formaldehyde resin. It was suggested that the low cross-linking density allowed for water-induced structural rearrangements in the incompletely cured melamine formaldehyde resin that increased the free space to accommodate water molecules at elevated RH [18]. A similar effect may also apply for the cured resins in the present study. It should be noted, however, that a further heat exposure of methylated DMDHEU to 160 °C for 48 h or a prolonged heat exposure for 100 h to 120 °C had nearly no effect on the MC at 95% RH. The MCs at 95% RH were 35.1% (120 °C, 48 h), 35.0% (120 °C, 100 h) and 34.9 (160 °C, 48 h). Therefore, the steep increase in MC at elevated RH was not an effect of an incomplete heat cure of the resins.
The sorption isotherms showed clear differences in absorption and desorption between the different N-methylol compounds (Fig. 5). The MC of cured DMDHEU remained below 25% MC, whereas cured mDMDHEU and mDMDHEU + DEG reached MCs of ca. 35 and 55% at 95% RH, respectively. Contrary to curing DMDHEU in cellulose fabrics or wood, pure DMDHEU is limited to self-condensation reactions that result in the formation of covalent bonds between adjacent DMDHEU monomers via the hydroxymethyl groups [7]. Methylated and diethyleneglycolated DMDHEU are well known for a reduced formaldehyde release that, however, is accompanied by a significantly lower reactivity compared to DMDHEU [47]. Therefore, resin formed from ether-modified DMDHEU is expected to have a lower cross-linking density compared to the use of DMDHEU. Therefore, resins based on mDMDHEU and mDMDHEU + DEG may be more prone to water-induced, structural rearrangements that lead to the enhanced moisture uptake at elevated RH. The differences in the sorption behavior of the different resins are also consistent with our observations on changes in their appearance during storage. Resins based on DMDHEU and diethyleneglycolated DMDHEU appeared hard and brittle immediately after curing at 120 °C. However, after several weeks of storage at 20 °C and 65% RH, the resin based on DMDHEU retained its shape and haptic, while the resin based on mDMDHEU + DEG changed to a gel-like consistency, presumably due to a lower cross-linking density. In addition, DEG remains in the wood structure after the cure of mDMDHEU + DEG, resulting in the presence of an additional, hygroscopic substance with further sorption sites in cured, diethyleneglycolated DMDHEU [7].
Water vapor sorption of treated wood
As explained above, the presence of the different resins inside the wooden structure changed the water mass (numerator) as well as the sample mass (denominator). For reasons of simplicity, only the corrected MC (MCR) is shown, which relates the mass of water to the dry wood mass. This correction of the reference sample mass by the corresponding WPG has previously been recommended for wood treated with DMDHEU [6].
The water vapor sorption behavior of untreated and resin modified wood particles was analyzed in the hygroscopic range (0–95% RH) by DVS measurements. Absorption isotherms of modified wood specimens (Fig. 6) differed significantly from the sorption isotherms of the cured resins (Fig. 5) and showed the typical IUPAC type II isotherm that is known for unmodified wood. Up to a RH of ca. 85%, all treated samples showed a higher MCR in comparison with the untreated reference sample, and this increase in MCR was larger after the treatment with a 20% solid content. This was in line with additional sorption sites in the treated wood shown by the HDX measurements and the moisture uptake by the cured resins. At elevated RH (> 85% RH), however, the differences in MCR between resin modified and the untreated reference sample decreased. The MCR of the samples that were treated at a 10% solid content even decreased below the reference MC. Therefore, there must have been a moisture exclusion mechanism in the treated wood that counterbalanced the moisture uptake of the resins. One possible mechanism is the reduced spatial availability for water in the treated wood due to the cell wall bulking. However, this seems to be contradicted by the treatment with a 20% solid content that caused an increase in MCR even though the dry dimensions of the treated wood blocks suggested a higher cell wall bulking.
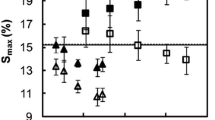
The effects of the treatments and the differences between the different cyclic N-methylol compounds became more obvious when looking at the course of the MCR ratios in the hygroscopic range (Fig. 7). The MCR ratio related the MCR of the treated samples to the MCR of the untreated reference sample at a given RH. At most RH steps, the MCR ratio was above 1, due to the additional moisture uptake by the resins. However, the MCR ratios decreased with increasing RH, particularly for wood treated with DMDHEU and methylated DMDHEU. A similar finding has been reported by [50] for wood treated with phenol or melamine formaldehyde resin. They suggested that the presence of the resins within the cell wall reduced the flexibility of the cell wall matrix, which restraint the swelling of the cell wall. However, this explanation is inconsistent with the almost negligible reduction in the water-saturated dimensions of the treated wood blocks after water-soaking cycles, which disconfirmed a significant swelling restraint of the treated wood. The same conclusion was drawn by [18], who explained the decreasing MCR ratio of wood treated with melamine formaldehyde resin by the low moisture uptake of the pure resin at elevated RH in contrast to the steep increase in the sorption isotherm of untreated wood. This explanation does not seem to be consistent with the present results either, due to the strong moisture uptake of the pure resins at elevated RH. However, the cyclic N-methylol compounds used in the present study are likely to react with the wood polymers by forming ether and hydrogen bonds. While this did not restraint the maximum swelling of the wood cell wall, the additional bond formation may have altered the structure of the resin significantly compared to the resin formed by self-condensation. The outcome may have been a more rigid resin structure that did not allow the steep increase in MC at elevated RH.
MCR ratios of wood as a function of the target relative humidity (%) based on the DVS measurement (filled symbols) and the measurement after storage over saturated salt solutions (half-filled symbols) for absorption (a–c) and desorption (d–f) of wood after treatment with DMDHEU (a, d), mDMDHEU (b, e) and mDMDHEU + DEG (c, f)
The combination of such an altered resin structure with a reduced spatial availability for water molecules by cell wall bulking may explain the reduction in MCR for wood treated with a 10% solid content below the reference MC at 95% RH, even though the MC of the cured resins exceeded this reference MC considerably. Treating the wood at a solid content of 20% further reduced the spatial availability for water, as indicated by an increasing CWB value, but may have resulted in excess resin so that self-condensation dominated over the co-condensation with the cell wall polymers. In particular, increasing the solid content of the impregnation solution may have resulted in larger quantities of resin that cured in the cell lumen via self-condensation. These resin deposits may have contributed to the moisture uptake significantly, without causing a moisture exclusion effect.
The DVS measurements were complemented by the long-term conditioning of small wood blocks over saturated salt solutions. Saturated solutions of sodium chloride and potassium sulfate were used, which should have resulted in RH levels of 75 and 97%, respectively [28]. However, the actual RH was not measured during the conditioning over the salt solutions. It is reasonable to assume that there have been slight deviations from the target RH levels, as explained in detail by [51], in particular due to small fluctuations in ambient temperature during the conditioning over the salt solutions. Hence, it remained unclear if deviations in absolute MCR values between the DVS analysis and the conditioning over salt solution were a material-inherent effect or the result of a difference in the actual RH. Consequently, MCR ratios were used to compare the effectiveness of the treatments in changing the sorption behavior of the wood during short-term (DVS) and long-term (salt solutions) conditioning.
Independent of the solid content or the N-methylol compound used, the MCR ratio measured after conditioning over salt solutions (Fig. 7a-c, half-filled symbols) was larger than the MCR ratio derived from the DVS measurements. Short holding times in the DVS (several hours) may not allow slow sorption processes, i.e., by mechanical relaxation, to be completed. This may especially apply to the treated wood, because the covalent bond formation between wood and resin may have further hindered the mechanical relaxation of the cell wall matrix. Therefore, the DVS measurements underestimated the MCR ratio of the treated wood to some extent. Nonetheless, the long-term conditioning over salt solutions confirmed the decrease in the MCR ratio with increasing RH as well as the lower MCR ratio for treatments with 10% solid content compared to a 20% solid content of the impregnation solution.
Conclusions
Dimensional changes of treated wood blocks suggested that the treatment caused a cell wall bulking as well as a swelling restraint by cross-linking of adjacent cell wall polymers. However, the swelling restraint was only observed directly after the treatment and disappeared in the course of several water-soaking cycles. Changes in the sorption behavior of the wood by the treatment with N-methylol compounds could not be fully explained by changes in the amount of sorption sites, or by the cell wall bulking effect that reduced the spatial availability for water in the treated cell walls. Treatments with N-methylol compounds at 10% solid content reduced the moisture content of wood at 95% RH, although the pure resins took up high quantities of moisture at elevated RH. It was speculated that the co-condensation of the N-methylol compounds with the wood polymers within the cell walls changed the sorption behavior compared to resin that was formed solely by self-condensation. However, the formation of covalent bonds between wood and resin may have also prolonged the time for the treated wood to attain an equilibrium moisture content. More studies are required to understand these cellular level chemical changes and their relevance for the properties of the treated wood.
References
Emmerich L, Bollmus S, Militz H (2019) Wood modification with DMDHEU (1.3-dimethylol-4.5-dihydroxyethyleneurea)—State of the art, recent research activities and future perspectives. Wood Mater Sci Eng 14:3–18
Emmerich L, Militz H, Brischke C (2020) Long-term performance of DMDHEU-treated wood installed in different test set-ups in ground, above ground and in the marine environment. Int Wood Prod J 11:27–37
Krause A (2006) Holzmodifizierung mit N-Methylolvernetzern. PhD thesis. University of Goettingen, Faculty of Forest Sciences, Goettingen
Bollmus S (2011) Biologische und technologische Eigenschaften von Buchenholz nach einer Modifizierung mit 1,3-dimethylol-4,5-dihydroxyethyleneurea (DMDHEU). PhD thesis. University of Goettingen, Faculty of Forest Sciences, Goettingen
Dieste A, Krause A, Militz H (2008) Modification of Fagus sylvatica (L.) with 1,3-dimethylol-4,5-dihydroxyethylene urea (DMDHEU): part 1. Estimation of heat adsorption by the isosteric method (Hailwood–Horrobin model) and by solution calorimetry. Holzforschung 62:577–583
Dieste A, Krause A, Mai C, Militz H (2010) The calculation of EMC for the analysis of wood/water relations in Fagus sylvatica L. modified with 1,3-dimethylol-4,5-dihydroxyethyleneurea. Wood Sci Technol 44:597–606
Schindler WD, Hauser PJ (2004) Chemical finishing of textiles. Woodhead Publishing Ltd, England, Cambridge
Andrews BA, Trask-Morrell BJ (1997) Long term formaldehyde emissions from DMDHEU-finished cotton fabrics. Text Chem Color 29:16–19
Paul R (ed) (2014) Functional finishes for textiles: improving comfort, performance and protection. Elsevier, Amsterdam
Dieste A, Krause A, Mai C, Sèbe G, Grelier S, Militz H (2009) Modification of Fagus sylvatica L. with 1,3-dimethylol-4,5-dihydroxy ethylene urea (DMDHEU). Part 2: pore size distribution determined by differential scanning calorimetry. Holzforschung 63:89–93
Yasuda R, Minato K, Norimoto M (1994) Chemical modification of wood by non-formaldehyde cross-linking reagents. Wood Sci Technol 28:209–218
Himmel S, Mai C (2015) Effects of acetylation and formalization on the dynamic water vapor sorption behavior of wood. Holzforschung 69:633–643
Thygesen LG, Engelund ET, Hoffmeyer P (2010) Water sorption in wood and modified wood at high values of relative humidity. Part I: results for untreated, acetylated, and furfurylated Norway spruce. Holzforschung 64:315–323
Beck G, Strohbusch S, Larnøy E, Militz H, Hill CAS (2017) Accessibility of hydroxyl groups in anhydride modified wood as measured by deuterium exchange and saponification. Holzforschung 72:17–23
Thybring EE, Piqueras S, Tarmian A, Burgert I (2020) Water accessibility to hydroxyls confined in solid wood cell walls. Cellulose 27:5617–5627
Krause A, Jones D, Van der Zee M, Militz H (2003) Interlace treatment—wood modification with N-methylol compounds. In: Proceedings of the first European conference on wood modification, Ghent, Belgium, pp 317–327
Emmerich L (2016) Holzmodifizierung von Kiefer (Pinus sylvestris L.) mit DMDHEU und modifizierten DMDHEU-Varianten im Vergleich. Master thesis, University of Goettingen, Faculty of Forest Sciences, Goettingen
Altgen M, Altgen D, Klüppel A, Rautkari L (2020) Effect of curing conditions on the water vapor sorption behavior of melamine formaldehyde resin and resin-modified wood. J Mater Sci 55:11253–11266. https://doi.org/10.1007/s10853-020-04814-0
EN 84 (1997) Wood preservatives—Accelerated ageing of treated wood prior to biological testing—Leaching procedure. European Committee for Standardization, Brussels
Hill CAS, Jones D (1996) The dimensional stabilisation of Corsican pine sapwood by reaction with carboxylic acid anhydrides. The effect of chain length. Holzforschung 50:457–462
Altgen M, Awais M, Altgen D, Klüppel A, Mäkelä M, Rautkari L (2020) Distribution and curing reactions of melamine formaldehyde resin in cells of impregnation-modified wood. Sci Rep 10:1–10
Hill CAS, Ramsay J, Keating B, Laine K, Rautkari L, Hughes M, Constant B (2012) The water vapour sorption properties of thermally modified and densified wood. J Mater Sci 47:3191–3197. https://doi.org/10.1007/s10853-011-6154-8
Popescu CM, Hill CAS (2013) The water vapour adsorption–desorption behaviour of naturally aged Tilia cordata Mill. wood. Polym Degrad Stab 98:1804–1813
Majka J, Czajkowski Ł, Olek W (2016) Effects of cyclic changes in relative humidity on the sorption hysteresis of thermally modified spruce wood. BioResources 11:5265–5275
Thybring EE, Kymäläinen M, Rautkari L (2018) Experimental techniques for characterising water in wood covering the range from dry to fully water-saturated. Wood Sci Technol 52:297–329
Glass SV, Boardman CR, Zelinka SL (2017) Short hold times in dynamic vapor sorption measurements mischaracterize the equilibrium moisture content of wood. Wood Sci Technol 51:243–260
Glass SV, Boardman CR, Thybring EE, Zelinka SL (2018) Quantifying and reducing errors in equilibrium moisture content measurements with dynamic vapor sorption (DVS) experiments. Wood Sci Technol 52:909–927
Greenspan L (1977) Humidity fixed points of binary saturated aqueous solutions. J Res Natl Bur Stand 81:89–96
Xie Y, Krause A, Mai C, Militz H, Richter K, Urban K, Evans PD (2005) Weathering of wood modified with the N-methylol compound 1,3-dimethylol-4,5-dihydroxyethyleneurea. Polym Degrad Stabil 89:189–199
Baishya P, Maji TK (2014) Studies on effects of different cross-linkers on the properties of starch-based wood composites. ACS Sustain Chem Eng 2:1760–1768
Faix O (1991) Classification of lignins from different botanical origins by FT-IR spectroscopy. Holzforschung 45:21–28
Pandey KK, Theagarajan KS (1997) Analysis of wood surfaces and ground wood by diffuse reflectance (DRIFT) and photoacoustic (PAS) Fourier transform infrared spectroscopic techniques. Holz Roh Werkst 55:383–390
Emmerich L, Militz H (2020) Study on the impregnation quality of rubberwood (Hevea brasiliensis Müll. Arg.) and English oak (Quercus robur L.) sawn veneers after treatment with 1,3-dimethylol-4,5-dihydroxyethyleneurea (DMDHEU). Holzforschung 74:362–371
Stamm AJ, Seborg RM (1936) Minimizing wood shrinkage and swelling. J Ind Eng Chem 28:1164–1169
Tomasino C (1992) Chemistry and technology of fabric preparation and finishing. Department of Textile Engineering, Chemistry and Science College of Textiles, North Carolina State University
Rowell RM, Dickerson JP (2014) Acetylation of wood. In: Schultz TP, Goodell B, Nicholas DD (eds) Deterioration and protection of sustainable biomaterials—American Chemical Society Symposium Series. Oxford University Press, Washington DC, pp 301–327
Tarkow H, Stamm AJ (1953) Effect of formaldehyde treatments upon the dimensional stabilization of wood. Forest Prod J 3:33–37
Biziks V, Andersons B, Sansonetti E, Andersone I, Militz H, Grinins J (2015) One-stage thermo-hydro treatment (THT) of hardwoods: an analysis of form stability after five soaking–drying cycles. Holzforschung 69:563–571
Čermák P, Rautkari L, Horáček P, Saake B, Rademacher P, Sablík P (2015) Analysis of dimensional stability of thermally modified wood affected by re-wetting cycles. BioResources 10:3242–3253
Wentzel M, Altgen M, Militz H (2018) Analyzing reversible changes in hygroscopicity of thermally modified eucalypt wood from open and closed reactor systems. Wood Sci Technol 52:889–907
Wepner F (2006) Entwicklung eines Modifizierungsverfahrens für Buchenfurniere (Fagus sylvatica L.) auf Basis von zyklischen N-Methylol-Verbindungen. PhD thesis. University of Goettingen, Faculty of Forest Sciences, Goettingen
Altgen M, Willems W, Hosseinpourpia R, Rautkari L (2018) Hydroxyl accessibility and dimensional changes of Scots pine sapwood affected by alterations in the cell wall ultrastructure during heat-treatment. Polym Degrad Stab 152:244–252
Willems W, Altgen M, Rautkari L (2020) A molecular model for reversible and irreversible hygroscopicity changes by thermal wood modification. Holzforschung 74:420–425
Popescu CM, Hill CAS, Curling S, Ormondroyd G, Xie Y (2014) The water vapour sorption behaviour of acetylated birch wood: how acetylation affects the sorption isotherm and accessible hydroxyl content. J Mater Sci 49:2362–2371. https://doi.org/10.1007/s10853-013-7937-x
Beck G, Hill CAS, Cocher PM, Alfredsen G (2019) Accessibility of hydroxyl groups in furfurylated wood at different weight percent gains and during Rhodonia placenta decay. Eur J Wood Wood Prod 77:953–955
Raheel M (1996) Modern textile characterization method. International fiber science and technology series. Taylor & Francis Inc, London
Choudhury AKR (2017) Principles of textile finishing. Woodhead Publishing, Cambridge
Stevens CV, Smith BF (1970) Crosslinking cotton cellulose with ethyleneurea derivatives having varying hydrogen-bonding capabilities. II. Accessibility determinations. J Appl Polym Sci 14:1691–1700
Rautkari L, Hill CAS, Curling S, Jalaludin Z, Ormondroyd G (2013) What is the role of the accessibility of wood hydroxyl groups in controlling moisture content? J Mater Sci 48:6352–6356. https://doi.org/10.1007/s10853-013-7434-2
Hosseinpourpia R, Adamopoulos S, Mai C (2015) Dynamic vapour sorption of wood and holocellulose modified with thermosetting resins. Wood Sci Technol 50:165–178
Strømdahl K (2000) Water Sorption in Wood and Plant Fibres. PhD thesis. Technical University of Denmarl, Kongens Lyngby
Acknowledgements
The authors acknowledge and cordially thank the Northern European Network for Wood Science and Engineering for granting the first author a travel fund for the research visit at Aalto University.
Funding
Open Access funding provided by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Stephen Eichhorn.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Emmerich, L., Altgen, M., Rautkari, L. et al. Sorption behavior and hydroxyl accessibility of wood treated with different cyclic N-methylol compounds. J Mater Sci 55, 16561–16575 (2020). https://doi.org/10.1007/s10853-020-05224-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-020-05224-y