Abstract
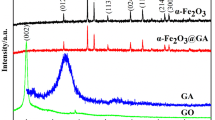
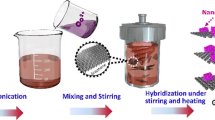
The fine shape control of metal oxides nanocrystal and component are the key for the preparation of high-performance metal oxides/graphene nanocomposites. Herein, a simpler and practicable in situ one-pot approach was used to synthetize α-Fe2O3@reduced graphene oxide (rGO) core–shell nanocomposites. By controlling the amount of hydrazine hydrate and GO in reaction system, the shape of α-Fe2O3 nanocrystals can be tailored from spindle gradually to ellipsoid and quasi-sphere, and the thickness of enwrapped rGO can also be finely controlled. The as-prepared α-Fe2O3@rGO core–shell composites showed much better lithium storage performance than bare α-Fe2O3 nanocrystals. Owing to the core–shell structure and the high conductivity and stability of rGO nanosheet, the quasi-sphere-α-Fe2O3@rGO composites delivered a high reversible specific capacity up to 971 mAh g−1 at the current density of 0.2 A g−1, retaining 530 mA h g−1 at 2 A g−1 and 361 mA h g−1 at 5 A g−1 after 800 cycles with only 0.05% decay per-cycle. Moreover, the facile approach can provide a new strategy for the fabrication of other shape-size dependent, functional, and multicomponent graphene-based composites.








Similar content being viewed by others
References
Yin J, Yu Z, Gao F, Wang J, Pang H, Lu Q (2010) Low-symmetry iron oxide nanocrystals bound by high-index facets. Angew Chem Int Ed 49:6328–6332
Zhou X, Lan J, Liu G, Deng K, Yang Y, Nie G, Yu J, Zhi L (2012) Facet-mediated photodegradation of organic dye over hematite architectures by visible light. Angew Chem Int Ed 51:178–182
Wu C, Xu Y, Ao L, Jiang K, Shang L, Li Y, Hu Z, Chu J (2020) Robust three-dimensional porous rGO aerogel anchored with ultra-fine α-Fe2O3 nanoparticles exhibit dominated pseudocapacitance behavior for superior lithium storage. J Alloys Compd 816:152627
Chen D, Zhou S, Quan H, Zou R, Gao W, Luo X, Guo L (2018) Tetsubo-like α-Fe2O3/C nanoarrays on carbon cloth as negative electrode for high-performance asymmetric supercapacitors. Chem Eng J 341:102–111
Frindy S, Sillanpää M (2020) Synthesis and application of novel α-Fe2O3/graphene for visible-light enhanced photocatalytic degradation of RhB. Mater Des 188:108461
Mazloum-Ardakani M, Sadri N, Eslami V (2020) Detection of dexamethasone sodium phosphate in blood plasma: application of hematite in electrochemical sensors. Electroanalysis 32:1148–1154
Liang Y, Wang M, Xiong J, Hou J, Wang X, Tan W (2019) Al-substitution-induced defect sites enhance adsorption of Pb2+ on hematite. Environ Sci Nano 6:1323–1331
Sivula K, Le Formal F, Grätzel M (2011) Solar water splitting: progress using hematite (α-Fe2O3) photoelectrodes. Chemsuschem 4:432–449
Mandal S, Müller AHE (2008) Facile route to the synthesis of porous α-Fe2O3 nanorods. Mater Chem Phys 111:438–443
Nasibulin AG, Rackauskas S, Jiang H, Tian Y, Mudimela PR, Shandakov SD, Nasibulina LI, Jani S, Kauppinen EI (2010) Simple and rapid synthesis of α-Fe2O3 nanowires under ambient conditions. Nano Res 2:373–379
Yang P, Ding Y, Lin Z, Chen Z, Li Y, Qiang P, Ebrahimi M, Mai W, Wong CP, Wang ZL (2014) Low-cost high-performance solid-state asymmetric supercapacitors based on MnO2 nanowires and Fe2O3 nanotubes. Nano Lett 14:731–736
Chen L, Yang X, Chen J, Liu J, Wu H, Zhan H, Liang C, Wu M (2010) Continuous shape- and spectroscopy-tuning of hematite nanocrystals. Inorg Chem 49:8411–8420
Xu L, Xia J, Wang L, Qian J, Li H, Wang K, Sun K, He M (2014) a-Fe2O3 cubes with high visible-light-activated photoelectrochemical activity towards glucose: hydrothermal synthesis assisted by a hydrophobic ionic liquid. Chemistry 20:2244–2253
Liu Z, Yu R, Dong Y, Li W, Lv B (2017) The adsorption behavior and mechanism of Cr(VI) on 3D hierarchical α-Fe2O3 structures exposed by (001) and non-(001) planes. Chem Eng J 309:815–823
Quan H, Cheng B, Xiao Y, Lei S (2016) One-pot synthesis of α-Fe2O3 nanoplates-reduced graphene oxide composites for supercapacitor application. Chem Eng J 286:165–173
Han S, Hu L, Liang Z, Wageh S, Al-Ghamdi AA, Chen Y, Fang X (2014) One-step hydrothermal synthesis of 2D hexagonal nanoplates of α-Fe2O3/graphene composites with enhanced photocatalytic activity. Adv Funct Mater 24:5719–5727
Chen D, Wei W, Wang R, Zhu J, Guo L (2012) α-Fe2O3 nanoparticles anchored on graphene with 3D quasi-laminated architecture: in situ wet chemistry synthesis and enhanced electrochemical performance for lithium ion batteries. New J Chem 36:1589–1595
Chen D, Quan H, Liang J, Guo L (2013) One-pot synthesis of hematite@graphene core@shell nanostructures for superior lithium storage. Nanoscale 5:9684
Meng F, Li J, Cushing SK, Bright J, Zhi M, Rowley JD, Hong Z, Manivannan A, Bristow AD, Wu N (2013) Photocatalytic water oxidation by hematite/reduced graphene oxide composites. ACS Catal 3:746–751
Xia H, Hong C, Li B, Zhao B, Lin Z, Zheng M, Savilov SV, Aldoshin SM (2015) Facile synthesis of hematite quantum-dot/functionalized graphene-sheet composites as advanced anode materials for asymmetric supercapacitors. Adv Funct Mater 25:627–635
Wu Z-S, Zhou G, Yin L-C, Ren W, Li F, Cheng H-M (2012) Graphene/metal oxide composite electrode materials for energy storage. Nano Energy 1:107–131
Qi X, Zhang HB, Xu J, Wu X, Yang D, Qu J, Yu ZZ (2017) Highly efficient high-pressure homogenization approach for scalable production of high-quality graphene sheets and sandwich-structured alpha-Fe2O3/graphene hybrids for high-performance lithium-ion batteries. ACS Appl Mater Interfaces 9:11025–11034
Qu J, Yin YX, Wang YQ, Yan Y, Guo YG, Song WG (2013) Layer structured alpha-Fe2O3 nanodisk/reduced graphene oxide composites as high-performance anode materials for lithium-ion batteries. ACS Appl Mater Interfaces 5:3932–3936
Kovtyukhova NI, Ollivier PJ, Martin BR, Mallouk TE, Chizhik SA, Buzaneva EV, Gorchinskiy AD (1999) Layer-by-layer assembly of ultrathin composite films from micron-sized graphite oxide sheets and polycations. Chem Mater 11:771–778
de Faria DLA, Venâncio Silva S, de Oliveira MT (1997) Raman microspectroscopy of some iron oxides and oxyhydroxides. J Raman Spectrosc 28:873–878
Bersani D, Lottici PP, Montenero A (1999) Micro-Raman investigation of iron oxide films and powders produced by sol–gel syntheses. J Raman Spectrosc 30:355–360
Giarola M, Mariotto G, Ajò D (2011) Micro-Raman investigations on inclusions of unusual habit in a commercial tanzanite gemstone. J Raman Spectrosc 43:556–558
Stankovich S, Dikin DA, Piner RD, Kohlhaas KA, Kleinhammes A, Jia Y, Wu Y, Nguyen ST, Ruoff RS (2007) Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 45:1558–1565
Selvakumar R, Kavitha S, Sathishkumar M, Swaminathan K (2008) Arsenic adsorption by polyvinyl pyrrolidone K25 coated cassava peel carbon from aqueous solution. J Hazard Mater 153:67–74
Dispenza C, Presti CL, Belfiore C, Spadaro G, Piazza S (2006) Electrically conductive hydrogel composites made of polyaniline nanoparticles and poly(N-vinyl-2-pyrrolidone). Polymer 47:961–971
Kiran Kumar K, Ravi M, Pavani Y, Bhavani S, Sharma AK, Narasimha Rao VVR (2011) Investigations on the effect of complexation of NaF salt with polymer blend (PEO/PVP) electrolytes on ionic conductivity and optical energy band gaps. Phys B Condens Matter 406:1706–1712
Singh M, Kumar V (2009) Preparation and characterization of melamine-formaldehyde-polyvinylpyrrolidone polymer resin for better industrial uses over melamine resins. J Appl Polym Sci 114:1870–1878
Wu SY-H, Yang K-C, Tseng C-L, Chen J-C, Lin F-H (2010) Silica-modified Fe-doped calcium sulfide nanoparticles for in vitro and in vivo cancer hyperthermia. J Nanopart Res 13:1139–1149
Ruan HD, Frost RL, Kloprogge JT, Duong L (2002) Infrared spectroscopy of goethite dehydroxylation: III. FT-IR microscopy of in situ study of the thermal transformation of goethite to hematite. Spectrochim Acta A 58:967–981
Bourlinos AB, Georgakilas V, Zboril R, Steriotis TA, Stubos AK, Trapalis C (2009) Aqueous-phase exfoliation of graphite in the presence of polyvinylpyrrolidone for the production of water-soluble graphenes. Solid State Commun 149:2172–2176
Yoon S, In I (2010) Role of poly(N-vinyl-2-pyrrolidone) as stabilizer for dispersion of graphene via hydrophobic interaction. J Mater Sci 46:1316–1321. https://doi.org/10.1007/s10853-010-4917-2
Van T-K, Cha HG, Nguyen CK, Kim S-W, Jung M-H, Kang YS (2012) Nanocystals of hematite with unconventional shape-truncated hexagonal bipyramid and its optical and magnetic properties. Cryst Growth Des 12:862–868
Zheng Y, Cheng Y, Wang Y, Bao F, Zhou L, Wei X, Zhang Y, Zheng Q (2006) Quasicubic α-Fe2O3 nanoparticles with excellent catalytic performance. J Phys Chem B 110:3093–3097
Larcher D, Masquelier C, Bonnin D, Chabre Y, Masson V, Leriche JB, Tarascon JM (2003) Effect of particle size on lithium intercalation into α-Fe2O3. J Electrochem Soc 150:A133–A139
Wu X-L, Guo Y-G, Wan L-J, Hu C-W (2008) α-Fe2O3 nanostructures: Inorganic salt-controlled synthesis and their electrochemical performance toward lithium storage. J Phys Chem C 112:16824–16829
Zhang W-M, Wu X-L, Hu J-S, Guo Y-G, Wan L-J (2008) Carbon coated Fe3O4 nanospindles as a superior anode material for lithium-ion batteries. Adv Funct Mater 18:3941–3946
Zhang M, Lei D, Yin X, Chen L, Li Q, Wang Y, Wang T (2010) Magnetite/graphene composites: microwave irradiation synthesis and enhanced cycling and rate performances for lithium ion batteries. J Mater Chem 20:5538–5543
Yang Z, Shen J, Archer LA (2011) An in situ method of creating metal oxide–carbon composites and their application as anode materials for lithium-ion batteries. J Mater Chem 21:11092–11097
Chen W, Li S, Chen C, Yan L (2011) Self-assembly and embedding of nanoparticles by in situ reduced graphene for preparation of a 3D graphene/nanoparticle aerogel. Adv Mater 23:5679–5683
Wang B, Chen JS, Wu HB, Wang Z, Lou XWD (2011) Quasiemulsion-templated formation of α-Fe2O3 hollow spheres with enhanced lithium storage properties. J Am Chem Soc 133:17146–17148
Hassan MF, Rahman MM, Guo ZP, Chen ZX, Liu HK (2010) Solvent-assisted molten salt process: a new route to synthesise α-Fe2O3/C nanocomposite and its electrochemical performance in lithium-ion batteries. Electrochim Acta 55:5006–5013
Cheng F, Huang K, Liu S, Liu J, Deng R (2011) Surfactant carbonization to synthesize pseudocubic α-Fe2O3/C nanocomposite and its electrochemical performance in lithium-ion batteries. Electrochim Acta 56:5593–5598
Zhang ZY, Liang JS, Zhang X, Yang WF, Dong XL, Jung YG (2020) Dominant pseudocapacitive lithium storage in the carbon-coated ferric oxide nanoparticles (Fe2O3@C) towards anode materials for lithium-ion batteries. Int J Hydrog Energy 45:8186–8197
Zhou W, Zhu J, Cheng C, Liu J, Yang H, Cong C, Guan C, Jia X, Fan HJ, Yan Q, Li CM, Yu T (2011) A general strategy toward graphene@metal oxide core-shell nanostructures for high-performance lithium storage. Energy Environ Sci 4:4954–4961
Acknowledgements
This work was supported by the Nation Natural Science Foundation (Grant No. 51968049) of China, the Youth Science Foundation (Grant No. 20192ACB21031) of Jiangxi Province, China, the Young Talents Training Plan (Grant No. 20192BCB23012) for Scientific and Technological Innovation of Jiangxi Province, China, and the Graduate Innovation Foundation (YC2018009) of Jiangxi Province, China.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Handling Editor: Joshua Tong.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10853_2020_5215_MOESM1_ESM.pdf
Additional digital photograph, AFM, Raman, SEM, TEM and electrochemical data. This material is available free of charge via the Internet (PDF 1368 kb)
Rights and permissions
About this article
Cite this article
Quan, H., Zeng, W., Pan, M. et al. Controlled synthesis of α-Fe2O3@rGO core–shell nanocomposites as anode for lithium ion batteries. J Mater Sci 56, 664–676 (2021). https://doi.org/10.1007/s10853-020-05215-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-020-05215-z