Abstract
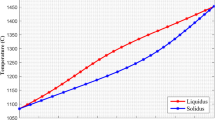
A modified thermodynamic approach to describe melting in isolated nanoscale materials is suggested. The Gibbs free energy change of nanoscale alloy particles is modeled as a function of composition, temperature and nucleus and particle sizes. Cu–Ni has been chosen as a model system due to the availability of thermodynamic data within the high-temperature interval 1300–1600 K. For the first time, “melting loops” in the temperature–composition phase diagram were calculated for nanoparticle of 25 and 80 nm, respectively. It is shown that such loops represent the equilibrium two-phase solid–liquid states and do not coincide with the limiting solubility curves—the solidus and the liquidus. This new finding leads to the “melting loop” concept concerning phase diagrams of nanoscale alloys introduced in this paper. It is found that Cu–Ni nanoparticles can melt in different ways, whereas the dominant transition mechanism is surface-induced melting that initiates from the surface and then proceeds toward the core region. The decrease in size causes also a change of the melting temperature, the temperature width of the phase transition, the solubility limit, the concentration width of the melting loop as well as a change of the shape and slope of the equilibrium curves of the two-phase region of the phase diagram. As expected, when the size of the nanoscale particle increases, the solidus temperature increases and the size-dependent phase diagram approaches the bulk phase diagram.






Similar content being viewed by others
References
Wilde G, Bunzel P, Roesner H, Weissmuller J (2007) Phase equilibria and phase diagrams of nanoscaled systems. J Alloys Compd 434:286–289. https://doi.org/10.1016/j.jallcom.2006.08.314
Guisbiers G, Mejia-Rosales S, Khanal S, Ruiz-Zepeda F, Whetten RL, Jose-Yacaman M (2014) Gold–Copper nano-alloy tumbaga in the era of nano-: phase diagram and segregation. Nano Lett 14:6718–6726. https://doi.org/10.1021/nl503584q
Liang LH, Liu D, Jiang Q (2003) Size-dependent continuous binary solution phase diagram. Nanotechnology 14:438–442. https://doi.org/10.1088/0957-4484/14/4/306
Wautelet M (1992) Effects of size, shape and environment on the phase diagrams of small structures. Nanotechnology 3:42–43. https://doi.org/10.1088/0957-4484/3/1/008
Park J, Lee J (2008) Phase diagram reassessment of Ag–Au system including size effect. Calphad 32:135–141. https://doi.org/10.1016/j.calphad.2007.07.004
Liu XJ, Wang CP, Jiang JZ, Ohnuma I, Kainuma R, Ishida K (2005) Thermodynamic calculation of phase diagram and phase stability with nano-size particle. Int J Mod Phys B 19:2645–2650. https://doi.org/10.1142/S0217979205031468
Tanaka T (2001) Thermodynamic evaluation of nano-particle binary alloy phase diagrams. Z METALLKD 92:1236–1241
Buffat Ph, Borel J-P (1976) Size effect on the melting temperature of gold particles. Phys Rev A 13:2287–2298. https://doi.org/10.1103/PhysRevA.13.2287
Kaptay G, Janczak-Rusch J, Pigozzi G, Jeurgens LPH (2014) Theoretical analysis of melting point depression of pure metals in different initial configurations. J Mater Eng Perform 23:1600–1607. https://doi.org/10.1007/s11665-014-0885-z
Weissmueller J, Bunzel P, Wilde G (2004) Two-phase equilibrium in small alloy particles. Scr Mater 51:813–818. https://doi.org/10.1016/jscriptamat2004060
Ulbricht H, Schmelzer J, Mahnke R, Schweitzer F (1988) Thermodynamics of finite systems and kinetics of first-order phase transitions. BSB Teubner, Leipzig. https://doi.org/10.1007/978-3-322-96427-4_3
Shirinyan AS, Wautelet M (2004) Phase separation in nano-particles. Nanotechnology 15:1720–1731. https://doi.org/10.1088/0957-4484/15/12/004
Qi WH, Wang MP, Zhou M, Shen XQ, Zhang XF (2006) Modeling cohesive energy and melting temperature of nanocrystals. J Phys Chem Solids 67:851–855
Safaei A (2011) Cohesive energy and physical properties of nanocrystals. Philos Mag 91:1509–1539. https://doi.org/10.1080/14786435.2010.548836
Shirinyan A (2015) Concept of size-dependent atomic interaction energies for solid nanomaterials:thermodynamic and diffusion aspects. Metallofiz Noveishie Tekhnol 37:475–486
Shirinyan A, Bilogorodskyy Y (2012) Atom–atom interactions in continuous metallic nanofilms. Phys Met Metall 13:823–830. https://doi.org/10.1134/S0031918X12090116
Kaptay G (2012) Nano-Calphad: extension of the Calphad method to systems with nano-phases and complexions. J Mater Sci 47:8320–8335. https://doi.org/10.1007/s10853-012-6772
Shirinyan A, Wilde G, Bilogorodskyy Y (2018) Solidification loops in the phase diagram of nanoscale alloy particles: from a specific example towards a general vision. J Mater Sci 53:2859–2879. https://doi.org/10.1007/s10853-017-1697-y
Shirinyan AS, Gusak M (2004) Phase diagram of decomposing nano-alloys. Philos Mag A 84:579–593
Shirinyan AS, Gusak AM, Wautelet M (2005) Phase diagram versus diagram of solubility: What is the difference for nano-systems? Acta Mater 53:5025–5032
Jesser WA, Shneck RZ, Gille WW (2004) Solid–liquid equilibria in nano-particles of Pb–Bi alloys. Phys Rev B 69:144121. https://doi.org/10.1103/PhysRevB.69.144121
Kofman R, Cheyssac P, Aouaj A, Lereah Y, Deutscher G, Ben-David T, Penisson J, Bourret A (1994) Surface melting enhanced by curvature effects. Surf Sci 303:231–246. https://doi.org/10.1016/0039-6028(94)90635-1
Dai C, Saidi P, Song H, Yao Z, Daymond MR, Hoyt JJ (2017) A test of a phenomenological model of size dependent melting in Au nanoparticles. Acta Mater 136:11–20. https://doi.org/10.1016/j.actamat.2017.06.052
Frenkenvan der Veen JWMJF (1985) Observation of surface melting. Phys Rev Lett 54:134–137. https://doi.org/10.1103/PhysRevLett.54.134
Theis W, Horn K (1995) Surface premelting in Al(110) observed by core-level photoemission. Phys Rev B 51:7157–7159. https://doi.org/10.1103/PhysRevB.51.7157
Ruan C-Y, Murooka Y, Raman RK, Murdick RA (2007) Dynamics of size selected gold nanoparticles studied by ultrafast electron nanocrystallography. Nano Lett 7:1290–1296. https://doi.org/10.1021/nl070269h
Fensin SJ, Olmsted D, Buta D, Asta M, Karma A, Hoyt JJ (2010) Structural disjoining potential for grain-boundary premelting and grain coalescence from molecular-dynamics simulations. Phys Rev E 81:031601. https://doi.org/10.1103/PhysRevE.81.031601
Barnett RN, Landman U (1991) Surface premelting of Cu(110). Phys Rev B 44:3226–3239. https://doi.org/10.1103/PhysRevB.44.3226
Wang N, Rokhlin SI, Farson DF (2008) Nonhomogeneous surface premelting of Au nanoparticles. Nanotechnology 19:415701. https://doi.org/10.1088/0957-4484/19/41/415701
Gusak AM, Kovalchuk AO, Straumal BB (2013) Interrelation of depletion and segregation in decomposition of nano-particles. Philos Mag A 93:1677–1689. https://doi.org/10.1080/14786435.2012.753481
Shirinyan AS (2015) Two-phase equilibrium states in individual CuNi nano-particles: size, depletion and hysteresis effects. Beilstein J Nanotechnol 6:1811–1820
Reshetenko TV, Avdeeva LB, Ismagilov ZR, Chuvilin AL, Ushakov VA (2003) Carbon capacious Ni–Cu–Al2O3 catalysts for methane decomposition. Appl Catal A 247:51–63. https://doi.org/10.1016/S0926-860X(03)00080
Huang SP, Balbuena PB (2002) Melting of bimetallic Cu–Ni Nano-clusters. J Phys Chem B 106:7225–7236
Guisbiers G, Khanal S, Ruiz-Zepeda F, Roque de la Puente J, Jose-Yacaman M (2014) Cu–Ni nano-alloy: mixed, core–shell or Janus nano-particle? Nanoscale 6:14630–14635
Sopousek J, Vrestal J, Pinkas J et al (2014) CuNi nano-alloy phase diagram Prediction and experiment. Calphad 45:33–39
Li G, Wang Q, Liu T, Wang K, He J (2010) Molecular dynamics simulation of the melting and coalescence in the mixed Cu–Ni nano-clusters. J Cluster Sci 21:45–55. https://doi.org/10.1007/s10876-010-0281-2
Shirinyan A, Wautelet M, Belogorodsky Y (2006) Solubility diagram of Cu–Ni nano-system. J Phys Condens Matter 18:2537–2551. https://doi.org/10.1088/0953-8984/18/8/016
Guisbiers G, Mendoza-Perez R (2017) Comment on phase stability and segregation behavior of nickel-based nanoalloys based on theory and simulation. J Alloys Compd 723:1079–1081
Steininger J (1970) Thermodynamics and calculation of the liquidus-solidus gap in homogenous monotonic alloy systems. J Appl Phys 41:2713–2724. https://doi.org/10.1063/1.1659286
Mey S (1992) Thermodynamic re-assessment of the Cu–Ni system. Calphad 16:255–260. https://doi.org/10.1016/0364-5916(92)90022-P
Matienseen W, Warlimont H (eds) (2005) Springer handbook of condensed matter and materials data. Springer, Berlin
Weast RC (eds) (1986–1987) CRC handbook of chemistry and physics, 67th edn. CRC Press, Inc Boca Raton, Florida
Shackelford JF, Alexander W (eds) (2001) CRC material science and engineering handbook, 3rd edn. CRC Press, New York
Smithells CJ, Brandes EA (eds) (1976) Metal reference book, 5th edn. Fulmer Research Ltd Butterworth, London and Boston
Jiang Q, Lu HM, Zhao M (2004) Modelling of surface energies of elemental crystals. J Phys Condens Matter 16:521–530. https://doi.org/10.1088/0953-8984/16/4/001
Magomedov MN (2004) Dependence of the surface energy on the size and shape of a nano-crystal. Phys Solid State 46:954–968
Hashimoto R, Shibuta Y, Suzuki T (2011) Estimation of solid–liquid interfacial energy from Gibbs–Thomson effect: a molecular dynamics study. ISIJ Int 51:1664–1667
Brillo J, Egry I, Giffard HS, Patti A (2004) Density and thermal expansion of liquid Au–Cu alloys. Int J Thermophys 25:1881–1888. https://doi.org/10.1007/s10765-004-7742-5
Lohoefer G, Brillo J, Egry I (2004) Thermophysical properties of undercooled liquid CuNi alloys. Int J Thermophys 25:1535–1550. https://doi.org/10.1007/s10765-005-0011
Xiao F, Yang R, Fang FL, Liu L, Zhao H (2008) Densities of molten Ni-(Cr Co, W) superalloys. Trans Nonferrous Met Soc China 18:24–27. https://doi.org/10.1016/S1003-6326(08)60005-9
Xiao F, Liang F, Nogi K (2005) Surface tension and molten Ni and Ni-Co alloys. J Mater Sci Technol 21:201–206
Turnbull D (1950) Formation of crystal nuclei in liquid metals. J Appl Phys 21:1022–1028
Jian Z, Kuribayashi K, Jie W (2002) Solid-liquid interface energy of metals at melting point and undercooled state. Mater Trans 43:721–726. https://doi.org/10.2320/matertrans.43.721
Tesfaye Firdu F, Taskinen P (2010) Densities of molten and solid alloys of (Fe, Cu, Ni, Co-S at Elevated Temperatures - Literature Review and Analysis, (Aalto University Publications in Materials Science and Engineering, Multiprint Oy, Espoo). https://doi.org/10.13140/2.1.2804.1282
Mills KC (2002) Recommended values of thermophysical properties for selected commercial alloys. Woodhead Publishing Limited & ASM International, Cambridge
Kaptay G (2015) Partial surface tension of components of a solution. Langmuir 31:5796–5804
Yeum KS, Speiser R, Poirier DR (1989) Estimation of the surface tensions of binary liquid alloys. Metall Trans B 20:693–703
Mei QS, Lu K (2007) Melting and superheating of crystalline solids: From bulk to nano-crystals. Prog Mater Sci 52:1175–1262
Dukarov S, Kryshtal A, Sukhov V (2015) Surface energy and wetting in island films. In: Aliofkhazraei M (eds) Wetting and Wettability. InTech, Rijeka, pp 169–206
Schamp CT, Jesser WA (2006) Two-phase equilibrium in individual nano-particles of Bi–Sn. Metall Mater Trans A 37a:1825–1829
Lee JG, Mori H, Yasuda H (2002) Alloy phase formation in nano-meter-sized particles in the In-Sn system. Phys Rev B 65:132106. https://doi.org/10.1103/PhysRevB65132106
Nam HS, Hwang NM, Yu BD, Yoon JK (2002) Formation of an icosahedral structure during the freezing of gold nano-clusters: surface-induced mechanism. Phys Rev Lett. 89: 275502. https://doi.org/10.1103/PhysRevLett.89.275502
Mottet C, Rossi G, Baletto F, Ferrando R (2005) Single impurity effect on the melting of nano-clusters. Phys Rev Lett 95:035501-1–035501-4. https://doi.org/10.1103/PhysRevLett.95.035501
Baletto F, Ferrando R (2005) Structural properties of nano-clusters: energetic, thermodynamic, and kinetic effects. Rev Mod Phys 77:371–423
Kim BJ, Tersoff J, Wen CY, Reuter MC, Stach EA, Ross FM (2009) Determination of size effects during the phase transition of a nanoscale Au–Si Eutectic. Phys Rev Lett 103:155701–155704. https://doi.org/10.1103/PhysRevLett.103.155701
Christian JW (1965) Theory of transformation in metals and alloys. Pergamon Press, New York
Chen SL, Daniel S, Zhang F et al (2002) The PANDAT software package and its applications. Calphad 26:175–188. https://doi.org/10.1016/S0364-5916(02)00034
Bale CW, Chartrand P, Degterov SA et al (2002) Fact Sage thermochemical software and databases. Calphad 26:189–228. https://doi.org/10.1016/jcalphad2016050
Davies RH, Dinsdale AT, Gisby JA, Robinson JAJ, Martin SM (2002) MTDATA—thermodynamic and phase equilibrium software from the national physical laboratory. CALPHAD 26:229–271. https://doi.org/10.1016/S0364-5916(02)00036-6
Gladgkikh MT (ed) (2004) Poverhnostnye javlenia I fazovye prevraschenia v condesirivanyh plenkah (Surface phenomena and phase transitions in condensed films). Kharkiv Karazin University, Kharkiv
Kelton KF (1991) Crystal nucleation in liquids and glasses. Solid State Phys 45:75–177. https://doi.org/10.1016/S0081-1947(08)60144-7
Acknowledgements
The authors thank DAAD (German Academic Exchange Service) for support of a German–Ukraine Projects (Ref. Codes 91730764 of Program 57440915 and 91617129 of Program 57210259).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shirinyan, A., Wilde, G. & Bilogorodskyy, Y. Melting loops in the phase diagram of individual nanoscale alloy particles: completely miscible Cu–Ni alloys as a model system. J Mater Sci 55, 12385–12402 (2020). https://doi.org/10.1007/s10853-020-04812-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-020-04812-2