Abstract
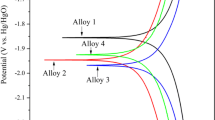
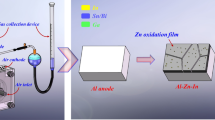
The electrochemical discharge behaviours of Al–0.5Mg–0.1Sn (wt%), Al–0.5Mg–0.1Sn–0.05In (wt%), Al–0.5Mg–0.1Sn–0.05Ga (wt%) and Al–0.5Mg–0.1Sn–0.05Ga–0.05In (wt%) alloys are investigated in 2 M NaCl solution. Based on electrochemical responses and microstructure observations, the influence mechanism of indium and gallium on the discharge behaviour of Al–Mg–Sn-based anode is clarified. The result indicates that Al–0.5Mg–0.1Sn–0.05Ga–0.05In (wt%) anode has the best discharge characteristics. Adding gallium accelerates active dissolution of Al–Mg–Sn anode. And adding indium leads to the appearance of discharge products (ie, In and In(OH)3), which inhibits the self-corrosion reaction of the anode. The peak power and peak energy density of Al–0.5Mg–0.1Sn–0.05Ga–0.05In (wt%) anodes reach approximately 92.96 mW cm−2 (at 140 mA cm−2) and 3385.4 W h kg−1 (at 20 mA cm−2) in 2 M NaCl solution, which increases by 447% and 104% compared with that of Al–0.5Mg–0.1Sn (wt%) anodes, respectively. Therefore, Al–Mg–Sn–Ga–In anodes could be a good and promising choice for high-speed discharge Al–air batteries in brine electrolytes.









Similar content being viewed by others
References
Wang L, Fu L, Li J, Zeng X, Xie H, Huang X, Wang H, Tang Y (2018) On an easy way to prepare highly efficient Fe/N-co-doped carbon nanotube/nanoparticle composite for oxygen reduction reaction in Al–air batteries. J Mater Sci 53:10280–10291. https://doi.org/10.1007/s10853-018-2245-0
Wu Z, Zhang H, Guo C, Zou J, Qin K, Ban C, Nagaumi H (2019) Effects of indium, gallium, or bismuth additions on the discharge behavior of Al–Mg–Sn-based alloy for Al–air battery anodes in NaOH electrolytes. J Solid State Electrochem 23:2483–2491
Li Q, Bjerrum N (2002) Aluminum as anode for energy storage and conversion: a review. J Power Sources 110:1–10
Zhang P, Liu X, Xue J, Jiang K (2020) The role of microstructural evolution in improving energy conversion of Al-based anodes for metal–air batteries. J Power Sources. https://doi.org/10.1016/j.jpowsour.2020.227806
Xue Y, Miao H, Li B, Sun S, Wang Q, Li S, Chen L, Liu Z (2017) Promoting effects of Ce0.75Zr0.25O2 on the La0.7Sr0.3MnO3 electrocatalyst for the oxygen reduction reaction in metal–air batteries. J Mater Chem A 5:6411–6415
Miao H, Wang Z, Wang Q, Sun S, Xue Y, Wang F, Zhao J, Liu Z, Yuan J (2018) A new family of Mn-based perovskite (La1-xYxMnO3) with improved oxygen electrocatalytic activity for metal–air batteries. Energy 154:561–570
Ryu J, Jang H, Park J, Yoo Y, Park M, Cho J (2018) Seed-mediated atomic-scale reconstruction of silver manganate nanoplates for oxygen reduction towards high-energy aluminium–air flow batteries. Nat Commun 9:1–10
Hopkins BJ, Shao-Horn Y, Hart DP (2018) Suppressing corrosion in primary aluminium–air batteries via oil displacement. Science 362:658–661
Ma J, Wen J, Gao J, Li Q (2014) Performance of Al–1Mg–1Zn–0.1Ga–0.1Sn as anode for Al–air battery. Electrochim Acta 129:69–75
Senel E, Nisancioglu K (2018) Effect of small concentrations of gallium and lead on anodic activation of aluminium in chloride solution. Corros Sci 131:330–339
Katsoufis P, Mylona V, Politis C, Avgouropoulos G, Lianos P (2020) Study of some basic operation conditions of an Al–air battery using technical grade commercial aluminum. J Power Sources. https://doi.org/10.1016/j.jpowsour.2019.227624
Gudić S, Radošević J, Smoljko I, Kliškić M (2005) Cathodic breakdown of anodic oxide film on Al and Al–Sn alloys in NaCl solution. Electrochim Acta 50:5624–5632
Kurt K, Diplas S, Walmsley JC, Nisancioglu K (2013) Effect of trace elements lead and tin on anodic activation of AA8006 aluminum sheet. J Electrochem Soc 160:C542–C552
Wu Z, Zhang H, Zou J, Shen X, Qin K, Ban C, Cui J, Nagaumi H (2020) Enhancement of the discharge performance of Al–0.5Mg–0.1Sn–0.05Ga (wt.%) anode for Al–air battery by directional solidification technique and subsequent rolling process. J Alloys Compd. https://doi.org/10.1016/j.jallcom.2020.154272
Flamini DO, Saidman SB, Bessone JB (2006) Aluminium activation produced by gallium. Corros Sci 48:1413–1425
Senel E, Nisancioglu K (2014) Role of dealloying on the electrochemical behaviour of aluminium alloyed with trace amounts of gallium. Corros Sci 85:436–444
Moghanni-Bavil-Olyaei H, Arjomandi J (2015) Performance of Al–1Mg–1Zn–0.1Bi–0.02In as anode for the Al–AgO battery. RSC Adv 5:91273–91279
Bessone JB, Flamini DO, Saidman SB (2005) Comprehensive model for the activation mechanism of Al–Zn alloys produced by indium. Corros Sci 47:95–105
Liang R, Su Y, Sui XL, Gu DM, Huang GS, Wang ZB (2019) Effect of Mg content on discharge behavior of Al–0.05Ga–0.05Sn–0.05Pb–xMg alloy anode for aluminium–air battery. J Solid State Electrochem 23:53–62
Li L, Liu H, Yan Y, Zhu H, Fang H, Luo X, Dai Y, Yu K (2019) Effects of alloying elements on the electrochemical behaviors of Al–Mg–Ga–In based anode alloys. Int J Hydrog Energy 44:12073–12084
Senel E, Nisancioglu K (2014) Anodic activation of aluminium containing small amounts of gallium and tin. Corros Sci 88:280–290
Nestoridi M, Pletcher D, Wood RJK, Wang S, Jones RL, Stokes KR, Wilcockc I (2008) The study of aluminium anodes for high power density Al/air batteries with brine electrolytes. J Power Sources 178:445–455
Srinivas M, Adapaka SK, Neelakantan L (2016) Solubility effects of Sn and Ga on the microstructure and corrosion behavior of Al–Mg–Sn–Ga alloy anodes. J Alloys Compd 683:647–653
Smoliko I, Gudić S, Kuzmanić N, Kliškić M (2012) Electrochemical properties of aluminium anodes for Al/air batteries with aqueous sodium chloride electrolyte. J Appl Electrochem 42:969–977
Gudić S, Smoliko I, Kliškić M (2010) Electrochemical behaviour of aluminium alloys containing indium and tin in NaCl solution. Mater Chem Phys 121:561–566
Gudić S, Smoliko I, Kliškić M (2010) The effect of small addition of tin and indium on the corrosion behavior of aluminium in chloride solution. J Alloys Compd 505:54–63
Ma J, Wen J, Gao J, Li Q (2014) Performance of Al–0.5Mg–0.02Ga–0.1Sn–0.5Mn as anode for Al–air battery in NaCl solutions. J Power Sources 253:419–423
Moghanni-Bavil-Olyaei H, Arjomandi J, Hosseini M (2017) Effects of gallium and lead on the electrochemical behavior of Al–Mg–Sn–Ga–Pb as anode of high rate discharge battery. J Alloys Compd 695:2637–2644
Ma J, Wen J, Zhu H, Li Q (2015) Electrochemical performances of Al–0.5Mg–0.1Sn–0.02In alloy in different solutions for Al–air battery. J Power Sources 293:592–598
Ma J, Wen J, Ren F, Wang G, Xiong Y (2016) Electrochemical performance of Al–Mg–Sn based alloys as anode for Al–Air battery. J Electrochem Soc 163:A1759–A1764
Fan L, Lu H, Leng J, Sun Z (2015) Performance of Al–0.6Mg–0.05Ga–0.1Sn–0.1In as anode for Al–air battery in KOH electrolytes. J Electrochem Soc 162:A2623–A2627
Yin X, Yu K, Zhang T, Fang H, Dai H, Xiong H, Dai Y (2017) Influence of rolling processing on discharge performance of Al–0.5Mg–0.1Sn–0.05Ga–0.05In alloy as anode for Al–air battery. Int J Electrochem Sci 12:4150–4163
Xiong H, Yin X, Yan Y, Dai Y, Fan S, Qiao X, Yu K (2016) Corrosion and discharge behaviors of Al–Mg–Sn–Ga–In in different solutions. J Mater Eng Perform 25:3456–3464
Sun Z, Lu H, Fan L, Hong Q, Leng J, Chen C (2015) Performance of Al–air batteries based on Al–Ga, Al–In and Al–Sn alloy electrodes. J Electrochem Soc 162:A2116–A2122
Li J, Zhang B, Wei Q, Wang N, Hou B (2017) Electrochemical behavior of Mg–Al–Zn–In alloy as anode materials in 3.5 wt.% NaCl solution. Electrochim Acta 238:156–167
Wang N, Wang R, Feng Y, Xiong W, Zhang J, Deng M (2016) Discharge and corrosion behaviour of Mg–Li–Al–Ce–Y–Zn alloy as the anode for Mg–air battery. Corros Sci 112:13–24
Wang N, Wang R, Peng C, Feng Y, Chen B (2012) Effect of hot rolling and subsequent annealing on electrochemical discharge behavior of AP65 magnesium alloy as anode for seawater activated battery. Corros Sci 64:17–27
Kaewmaneekul T, Lothongkum G (2013) Effect of aluminium on the passivation of zinc–aluminium alloys in artificial seawater at 80 °C. Corros Sci 66:67–77
Khireche S, Boughrara D, Kadri A, Hamadou L, Benbrahim N (2014) Corrosion mechanism of Al, Al–Zn and Al–Zn–Sn alloys in 3wt.% NaCl solution. Corros Sci 87:504–516
Wang N, Wang R, Peng C, Peng B, Feng Y, Hu C (2014) Discharge behaviour of Mg–Al–Pb and Mg–Al–Pb–In alloys as anodes for Mg–air battery. Electrochim Acta 149:193–205
Zhao M, Schmutz P, Brunner S, Liu M, Song G, Atrens A (2009) An exploratory study of the corrosion of Mg alloys during interrupted salt spray testing. Corros Sci 51:1277–1292
Cao D, Wu L, Sun Y, Wang G, Lv Y (2008) Electrochemical behavior of Mg–Li, Mg–Li–Al and Mg–Li–Al–Ce in sodium chloride solution. J Power Sources 177:624–630
Xiong H, Yu K, Yin X, Dai Y, Yan Y, Zhu H (2016) Effects of microstructure on the electrochemical discharge behavior of Mg–6wt%Al–1wt%Sn alloy as anode for Mg–air primary battery. J Alloys Compd 708:652–661
Brug GJ, Eeden ALG, Sluyters-Rehbach M, Sluyters JH (1984) The analysis of electrode impedances complicated by the presence of a constant phase element. J Electroanal Chem Interfacial Electrochem 176:275–295
Ma J, Ren F, Wang G, Xiong Y, Li Y, Wen J (2017) Electrochemical performance of melt-spinning Al–Mg–Sn based anode alloys. Int J Hydrog Energy 42:11654–11661
Liu X, Xue J, Zhang P, Wang Z (2019) Effects of the combinative Ca, Sm and La additions on the electrochemical behaviors and discharge performance of the as-extruded AZ91 anodes for Mg–air batteries. J Power Sources 414:174–182
Daniel C, Besenhard JO (2012) Handbook of battery materials. Wiley, Weinheim
Park IJ, Choi SR, Kim JG (2017) Aluminum anode for aluminium–air battery-part II: influence of In addition on the electrochemical characteristics of Al–Zn alloy in alkaline solution. J Power Sources 357:47–55
Andrei M, Gabriele F, Bonora PL, Scantlebury D (2003) Corrosion behaviour of magnesium sacrificial anodes in tap water. Mater Corros 54:5–11
Wang Q, Miao H, Xue Y, Sun S, Li S, Liu Z (2017) Performances of an Al–0.15Bi–0.15Pb–0.035Ga alloy as an anode for Al–air batteries in neutral and alkaline electrolytes. RSC Adv 7:25838–25847
Fan L, Lu H, Leng J (2015) Performance of fine structured aluminum anodes in neutral and alkaline electrolytes for Al–air batteries. Electrochim Acta 165:22–28
Cho YJ, Park IJ, Lee HJ, Kim JG (2015) Aluminum anode for aluminum–air battery-part I: influence of aluminum purity. J Power Sources 277:370–378
Liu X, Zhang P, Xue J (2019) The role of micro-naoscale AlSb precipitates in improving the discharge performance of Al–Sb alloy anodes for Al–air batteries. J Power Sources 425:186–194
Thomas JH (1977) ESCA study of the passive layer on Sn–Ni alloy. J Vac Sci Technol 14:1168–1172
Cossu G, Ingo GM, Mattogno G, Padeletti G, Proietti GM (1992) XPS investigation on vacuum thermal desorption of UV/ozone treated GaAs(100) surfaces. Appl Surf Sci 56–58:81–88
Iwakuro H, Tatsuyama C, Ichimura S (1982) XPS and AES studies on the oxidation of layered semiconductor. J Appl Phys 21:94–99
Graver B, Helvoort ATJV, Nisancioglu K (2010) Effect of heat treatment on anodic activation of aluminium by trace element indium. Corros Sci 52:3774–3781
Venugopal A, Raja VS (1997) The self regulating nature of In on the potential of Al in 3.5% NaCl solution. Corros Sci 39:1285–1289
Flamini DO, Saidman SB (2012) Electrochemical behaviour of Al–Zn–Ga and Al–In–Ga alloys in chloride media. Mater Chem Phys 136:103–111
Flamini DO, Saidman SB (2008) Polarisation behaviour of Al–Zn–Ga alloy in chloride medium. J Appl Electrochem 38:663–668
Acknowledgements
The authors gratefully acknowledge the support of the National Natural Science Foundation of China (Grant No. U1864209).
Funding
This study was funded by National Natural Science Foundation of China (Grant Number U1864209).
Author information
Authors and Affiliations
Contributions
ZW: Conceptualization, methodology, software, investigation, writing-original draft. HZ: Validation, formal analysis, visualization, project administration, resources, writing-review and editing, funding acquisition. KQ: validation, formal analysis, visualization. JZ: resources, writing-review and editing, supervision, data curation. KQ: resources, writing-review and editing, supervision, data curation. CB: writing-review and editing. JC: writing-review and editing. HN: writing-review and editing, funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
Author Haitao Zhang, Hiromi Nagaumi and Zibin Wu have received research grants from National Natural Science Foundation of China. No conflict of interest exits in the submission of this manuscript, and manuscript is approved by all authors for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wu, Z., Zhang, H., Qin, K. et al. The role of gallium and indium in improving the electrochemical characteristics of Al–Mg–Sn-based alloy for Al–air battery anodes in 2 M NaCl solution. J Mater Sci 55, 11545–11560 (2020). https://doi.org/10.1007/s10853-020-04755-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-020-04755-8