Abstract
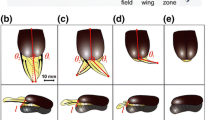
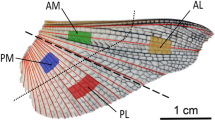
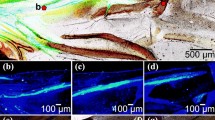
The deployable hind wings of Coleoptera are a highly specialized motive system that can fold and unfold in a unique way. Resilin in the wing membrane of Asian ladybird beetle (Harmonia axyridis) hind wings plays an active role during folding and unfolding of the wing. This study investigates the tensile properties of the hind wing and the distribution of resilin through the hind wing in an adult H. axyridis (Coleoptera: Coccinellidae) and how the resilin in the membrane of the hind wing affects its mechanical characteristics. The cross sections of veins of the hind wing are investigated by inverted fluorescence microscopy. Based on those results, two three-dimensional finite element models of the hind wing with/without resilin are established. The displacements, when subjected to pressure on the ventral side, are analyzed when the membrane wings are filled with/without resilin. The resilin in the hind wing is effectively for changing the flight performance such as the condition of stress and deformation. The results in this paper reveal the multiple functions of the resilin in the hind wings and have important implications for the design of biomimetic deployable micro-air vehicles.









Similar content being viewed by others
References
Geisler T (2016) Observations and measurements of wing parameters of the selected beetle species and the design of a mechanism structure implementing a complex wing movement. Int J Appl Mech Eng 21:837–847. https://doi.org/10.1515/ijame-2016-0049
Takizawa K, Tezduyar TE, Buscher A (2015) Space–time computational analysis of MAV flapping-wing aerodynamics with wing clapping. Comput Mech 55:1131–1141. https://doi.org/10.1007/s00466-014-1095-0
Phan HV, Au TKL, Park HC (2016) Clap-and-fling mechanism in a hovering insect-like two-winged flapping-wing micro air vehicle. R Soc Open Sci 3:160746. https://doi.org/10.1098/rsos.160746
Phan HV, Kang T, Park HC (2017) Design and stable flight of a 21 g insect-like tailless flapping wing micro air vehicle with angular rates feedback control. Bioinspir Biomim 12:036006. https://doi.org/10.1088/1748-3190/aa65db
Yan X, Qi M, Lin L (2015) Self-lifting artificial insect wings via electrostatic flapping actuators. In: 2015 28th IEEE international conference on micro electro mechanical systems (MEMS). IEEE, pp 22–25. https://doi.org/10.1109/memsys.2015.7050876
Nguyen Q-V, Chan WL, Debiasi M (2016) Hybrid design and performance tests of a hovering insect-inspired flapping-wing micro aerial vehicle. J Bionic Eng 13:235–248. https://doi.org/10.1016/S1672-6529(16)60297-4
Van Truong T, Nguyen Q-V, Lee H (2017) Bio-inspired flexible flapping wings with elastic deformation. Aerospace 4:37. https://doi.org/10.3390/aerospace4030037
Truong QT, Argyoganendro BW, Park HC (2014) Design and demonstration of insect mimicking foldable artificial wing using four-bar linkage systems. J Bionic Eng 11:449–458. https://doi.org/10.1016/S1672-6529(14)60057-3
Muhammad A, Park HC, Hwang DY et al (2009) Mimicking unfolding motion of a beetle hind wing. Chin Sci Bull 54:2416–2424. https://doi.org/10.1007/s11434-009-0242-z
Jitsukawa T, Adachi H, Abe T et al (2017) Bio-inspired wing-folding mechanism of micro air vehicle (MAV). Artif Life Robot 22:203–208. https://doi.org/10.1007/s10015-016-0339-9
Maes S, Massart X, Grégoire JC, De Clercq P (2014) Dispersal potential of native and exotic predatory ladybirds as measured by a computer-monitored flight mill. Biocontrol 59:415–425. https://doi.org/10.1007/s10526-014-9576-9
Miller LA, Peskin CS (2009) Flexible clap and fling in tiny insect flight. J Exp Biol 212:3076–3090. https://doi.org/10.1242/jeb.028662
Haas F, Beutel RG (2001) Wing folding and the functional morphology of the wing base in Coleoptera. Zoology 104:123–141. https://doi.org/10.1078/0944-2006-00017
Saito K, Nomura S, Yamamoto S et al (2017) Investigation of hindwing folding in ladybird beetles by artificial elytron transplantation and microcomputed tomography. Proc Natl Acad Sci 114:5624–5628. https://doi.org/10.1073/pnas.1620612114
Kwok K, Pellegrino S (2013) Folding, stowage, and deployment of viscoelastic tape springs. AIAA J 51:1908–1918. https://doi.org/10.2514/1.J052269
Schieber G, Born L, Bergmann P et al (2017) Hindwings of insects as concept generator for hingeless foldable shading systems. Bioinspir Biomim 13:016012. https://doi.org/10.1088/1748-3190/aa979c
Lawrence JF (1993) Evolution of the hind wing in coleoptera. Can Entomol 125:181–258. https://doi.org/10.4039/Ent125181-2
Ha NS, Truong QT, Goo NS, Park HC (2013) Biomechanical properties of insect wings: the stress stiffening effects on the asymmetric bending of the Allomyrina dichotoma beetle’s hind wing. PLoS ONE. https://doi.org/10.1371/journal.pone.0080689
Saito K, Tachi T, Niiyama R, Kawahara Y (2017) Design of a beetle inspired deployable wing. In: 41st mechanisms and robotics conference. ASME, vol 5B(4), pp 1–6. https://doi.org/10.1115/detc2017-67697
Sun J, Liu C, Bhushan B et al (2018) Effect of microtrichia on the interlocking mechanism in the Asian ladybeetle, Harmonia axyridis (Coleoptera: Coccinellidae). Beilstein J Nanotechnol 9:812–823. https://doi.org/10.3762/bjnano.9.75
Hedrick TL, Combes SA, Miller LA (2015) Recent developments in the study of insect flight. Can J Zool 93:925–943. https://doi.org/10.1139/cjz-2013-0196
Combes SA (2010) Materials, structure, and dynamics of insect wings as bioinspiration for MAVs. In: Blockley R, Shyy W (eds) Encyclopedia of aerospace engineering. Wiley, Chichester, pp 1–10. https://doi.org/10.1002/9780470686652.eae404
Fedorenko DN (2015) Transverse folding and evolution of the hind wings in beetles (Insecta, Coleoptera). Biol Bull Rev 5:71–84. https://doi.org/10.1134/S2079086415010028
Saito K, Yamamoto S, Maruyama M, Okabe Y (2014) Asymmetric hindwing foldings in rove beetles. Proc Natl Acad Sci 111:16349–16352. https://doi.org/10.1073/pnas.1409468111
Haas F (2006) Evidence from folding and functional lines of wings on inter-ordinal relationships in Pterygota. Arthropod Syst Phylogeny 64:149–158
Haas F, Gorb S, Blickhan R (2000) The function of resilin in beetle wings. Proc R Soc Lond Ser B Biol Sci 267:1375–1381. https://doi.org/10.1098/rspb.2000.1153
Rajabi H, Shafiei A, Darvizeh A, Gorb SN (2016) Resilin microjoints: a smart design strategy to avoid failure in dragonfly wings. Sci Rep 6:39039. https://doi.org/10.1038/srep39039
Burrows M (2016) Development and deposition of resilin in energy stores for locust jumping. J Exp Biol 219:2449–2457. https://doi.org/10.1242/jeb.138941
Su RS, Kim Y, Liu JC (2014) Resilin: protein-based elastomeric biomaterials. Acta Biomater 10:1601–1611. https://doi.org/10.1016/j.actbio.2013.06.038
Haas F, Gorb S, Wootton R (2000) Elastic joints in dermapteran hind wings: materials and wing folding. Arthropod Struct Dev 29:137–146. https://doi.org/10.1016/S1467-8039(00)00025-6
Li L, Guo C, Li X et al (2017) Microstructure and mechanical properties of rostrum in Cyrtotrachelus longimanus (Coleoptera: Curculionidae). Anim Cells Syst (Seoul) 21:199–206. https://doi.org/10.1080/19768354.2017.1330764
Andersen SO, Weis-Fogh T (1964) Resilin. A rubberlike protein in arthropod cuticle. In: Beament JWL, Treherne JE, Wigglesworth VB (eds) Advances in insect physiology. Academic Press, London, pp 1–65. https://doi.org/10.1016/s0065-2806(08)60071-5
Rajabi H, Ghoroubi N, Stamm K et al (2017) Dragonfly wing nodus: a one-way hinge contributing to the asymmetric wing deformation. Acta Biomater 60:330–338. https://doi.org/10.1016/j.actbio.2017.07.034
Mamat-Noorhidayah, Yazawa K, Numata K, Norma-Rashid Y (2018) Morphological and mechanical properties of flexible resilin joints on damselfly wings (Rhinocypha spp.). PLoS One 13:e0193147. https://doi.org/10.1371/journal.pone.0193147
Manuscript A (2012) Recombinant exon-encoded resilins for elastomeric biomaterials. Biomaterials 32:9231–9243. https://doi.org/10.1016/j.biomaterials.2011.06.010.Recombinant
Appel E, Heepe L, Lin CP, Gorb SN (2015) Ultrastructure of dragonfly wing veins: composite structure of fibrous material supplemented by resilin. J Anat 227:561–582. https://doi.org/10.1111/joa.12362
Sun J, Song Z, Pan C, Liu Z (2018) Analysis of light-mass and high-strength veins of hind wing from Asian Ladybird beetle. In: 2018 IEEE international conference on manipulation, manufacturing and measurement on the nanoscale (3M-NANO). IEEE, pp 142–145. https://doi.org/10.1109/3m-nano.2018.8552182
Neff D, Frazier SF, Quimby L et al (2000) Identification of resilin in the leg of cockroach, Periplaneta americana: confirmation by a simple method using pH dependence of UV fluorescence. Arthropod Struct Dev 29:75–83. https://doi.org/10.1016/S1467-8039(00)00014-1
Kreuz P, Arnold W, Kesel AB (2001) Acoustic microscopic analysis of the biological structure of insect wing membranes with emphasis on their waxy surface. Ann Biomed Eng 29:1054–1058. https://doi.org/10.1114/1.1424921
Peisker H, Michels J, Gorb SN (2013) Evidence for a material gradient in the adhesive tarsal setae of the ladybird beetle Coccinella septempunctata. Nat Commun 4:1607–1608. https://doi.org/10.1038/ncomms2576
Hou D, Zhong Z, Yin Y et al (2017) The role of soft vein joints in dragonfly flight. J Bionic Eng 14:738–745. https://doi.org/10.1016/S1672-6529(16)60439-0
Saha R, Nix WD (2002) Effects of the substrate on the determination of thin film mechanical properties by nanoindentation. Acta Mater 50:23–38. https://doi.org/10.1016/S1359-6454(01)00328-7
Bell TJ, Field JS, Swain MV (1992) Elastic–plastic characterization of thin films with spherical indentation. Thin Solid Films 220:289–294. https://doi.org/10.1016/0040-6090(92)90587-2
Betts CR (2009) The comparative morphology of the wings and axillae of selected Heteroptera. J Zool 1:255–282. https://doi.org/10.1111/j.1096-3642.1986.tb00639.x
Haas F, Wootton RJ (1996) Two basic mechanisms in insect wing folding. Proc R Soc Lond Ser B Biol Sci 263:1651–1658. https://doi.org/10.1098/rspb.1996.0241
Haas F (2000) Wing folding in insects: a natural, deployable structure. https://doi.org/10.1007/978-94-015-9514-8_15
Forbes WTM (1926) The wing folding patterns of the Coleoptera. J N Y Entomol Soc 34:91–139. https://doi.org/10.2307/25004114
Wootton RJ, Herbert RC, Young PG, Evans KE (2003) Approaches to the structural modelling of insect wings. Philos Trans R Soc B Biol Sci 358:1577–1587. https://doi.org/10.1098/rstb.2003.1351
Michels J, Gorb SN (2012) Detailed three-dimensional visualization of resilin in the exoskeleton of arthropods using confocal laser scanning microscopy. J Microsc 245:1–16. https://doi.org/10.1111/j.1365-2818.2011.03523.x
Jongerius SR, Lentink D (2010) Structural analysis of a dragonfly wing. Exp Mech 50:1323–1334. https://doi.org/10.1007/s11340-010-9411-x
Kantor Y, Kardar M, Nelson DR (1987) Tethered surfaces: statics and dynamics. Phys Rev A 35:3056–3071. https://doi.org/10.1103/PhysRevA.35.3056
Meresman Y, Ribak G (2017) Allometry of wing twist and camber in a flower chafer during free flight: how do wing deformations scale with body size? R Soc Open Sci. https://doi.org/10.1098/rsos.171152
Herbert RC, Young PG, Smith CW et al (2000) The hind wing of the desert locust (Schistocerca gregaria Forskål) II. Mechanical properties and functioning of the membrane C. J Exp Biol 203:2945–2955
Rothschild M, Von Euw J, Reichstein T (1972) Aristolochic acids stored by Zerynthia polyxena (Lepidoptera). Insect Biochem 2:334–343. https://doi.org/10.1016/0020-1790(72)90038-8
Chimakurthi SK, Cesnik CES, Stanford BK (2011) Flapping-wing structural dynamics formulation based on a corotational shell finite element. AIAA J 49:128–142. https://doi.org/10.2514/1.J050494
Faber JA, Arrieta AF, Studart AR (2018) Bioinspired spring origami. Science 359:1386–1391. https://doi.org/10.1126/science.aap7753
Weis-Fogh T (1961) Molecular interpretation of the elasticity of resilin, a rubber-like protein. J Mol Biol 3:648–667. https://doi.org/10.1016/S0022-2836(61)80028-4
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Number 31672348), Joint Fund for Pre-research of Equipment and Weapons Industry (Grant Number 6141B012833), China-EU H2020 FabSurfWAR Project (Grant Number 644971), and 111 Project (B16020) of China.
Author information
Authors and Affiliations
Contributions
ZLS and JYS designed the study; ZLS and YWY coordinated the study; ZLS, JT, and JYS conducted the research and analyzed the data; ZLS wrote the manuscript; JYS reviewed the manuscript, discussed the results, and gave the final approval for publication; Mr. Zhiqiang Zhang, ShenYang YuanJie Optics Technology Co., Ltd., offered technology supporting.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare there are no conflicts of interest to disclose.
Ethical standard
This work complies with ethical guidelines at Jilin University.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Song, Z., Yan, Y., Tong, J. et al. Asian ladybird folding and unfolding of hind wing: biomechanical properties of resilin in affecting the tensile strength of the folding area. J Mater Sci 55, 4524–4537 (2020). https://doi.org/10.1007/s10853-019-04326-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-019-04326-6