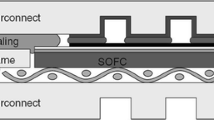
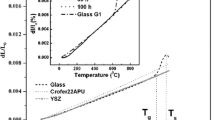
Abstract
In this work, we synthesized the glasses of the SiO2–Al2O3–CaO–Na2O–MgO–K2O–B2O3–Y2O3 system and investigated their thermal properties under changes in the ratios between the components in both modifying (MgO/CaO) and glass-forming (SiO2/B2O3) oxides. In addition, the effects of crystallization on both the properties of sealants and their behaviour at the boundary with ceramic yttria-stabilized zirconia (YSZ) electrolyte have been studied. X-ray diffraction analysis was used to determine the phase composition of the glasses both after quenching, dilatometric measurements and sealing of the glasses with the YSZ ceramic support. The thermal expansion coefficients (CTEs) found for the glasses and glass–ceramics are shown to be in good agreement with those of other cell components. The sealing temperatures of these materials are found to be lower than the operating temperature limit of the interconnector alloy. It is shown that Mg-rich glasses exhibit higher resistance to interaction with Cr compared to other compositions under study. The scanning electron microscopy and energy dispersive spectroscopy studies have confirmed the formation of gas-tight connections by the sealants under investigation. The glass demonstrating the highest stability in contact with the Crofer22APU alloy has been tested as a sealant for a tubular cell. This material is established to ensure a tight connection without cracks during operation at 850 °C for 250 h. The materials are thus found to be promising candidates for use as sealants for solid oxide fuel cells (SOFCs).










Similar content being viewed by others
References
Höland W, Beall G (2012) Class-ceramic technology. Wiley, Hoboken
Smeacetto F, Miranda A, Chrysanthou A et al (2014) Novel glass-ceramic composition as sealant for SOFCs. J Am Ceram Soc 97:3835–3842
Gurbinder K (2016) Solid oxide fuel cell components. Springer International Publishing, Switzerland
Mahato N, Banerjee A, Gupta A et al (2015) Progress in material selection for solid oxide fuel cell technology: a review. Prog Mater Sci 72:141–337
Lessing PA (2007) A review of sealing technologies applicable to solid oxide electrolysis cells. J Mater Sci 42:3465–3476. https://doi.org/10.1007/s10853-006-0409-9
Maharapta MK, Lu K (2010) Glass-based seals for solid oxide fuel and electrolyzer cells—a review. Mater Sci Eng, R 67:65–85
Ley KL, Krumplet M, Kumar R et al (1996) Glass-ceramic sealants for solid oxide fuel cells: part I. Physical properties. J Mater Res 11:1489–1493
Eichler K, Solow G, Otschik P et al (1999) BAS (BaO·Al2O3·SiO2)-glasses for high temperature applications. J Eur Ceram Soc 19:1101–1104
Sohn SB, Choi SY (2004) Suitable glass-ceramic sealant for planar solid-oxide fuel cells. J Am Ceram Soc 87:254–260
Larsen PH, James PF (1998) Chemical stability of MgO/CaO/Cr2O3–Al2O3–B2O3–phosphate glasses in solid oxide fuel cell environment. J Mater Sci 33:2499–2507. https://doi.org/10.1023/A:1004332614379
Nielsen KA, Solvang M, Nielsen SBL et al (2007) Glass composite seals for SOFC application. J Eur Ceram Soc 27:1817–1822
Chou YS, Stewenson JW, Choi JP (2013) Evaluation of a single cell and candidate materials with high water content hydrogen in a generic solid oxide fuel cell stack test fixture, part II: materials and interface characterization. Int J Appl Ceram Technol 10:97–106
Donald IW, Mallison PM, Metcalfe BL et al (2011) Recent developments in the preparation, characterization and applications of glass- and glass–ceramic-to-metal seals and coatings. J Mater Sci 46:1975–2000. https://doi.org/10.1007/s10853-010-5095-y
Reddy AA, Tulyaganov DU, Pascular MJ et al (2013) Diopside–Ba disilicate glass–ceramic sealants for SOFCs: enhanced adhesion and thermal stability by Sr for Ca substitution. Int J Hydrogen Energy 38:3073–3086
Smeacetto F, Salvo M, Ferraris M, Casalegno V, Asinari P, Chrysanthou A (2008) Characterization and performance of glass–ceramic sealant to join metallic interconnects to YSZ and anode-supported-electrolyte in planar SOFCs. J Eur Chem Soc 28:2521–2527
Smeacetto F, Salvo M, Ferraris M, Casalegno V, Asinari P (2008) Glass and composite seals for the joining of YSZ to metallic interconnect in solid oxide fuel cells. J Eur Chem Soc 28:611–616
Smeacetto F, Salvo M, Ferraris M, Cho J, Boccaccini AR (2008) Glass–ceramic seal to join Crofer 22 APU alloy to YSZ ceramic in planar SOFCs. J Eur Chem Soc 28:61–68
Smeacetto F, Chrysanthou A, Salvo M, Zhang Z, Ferraris M (2009) Performance and testing of glass-ceramic sealant used to join anode-supported-electrolyte to Crofer22APU in planar solid oxide fuel cells. J Power Sources 190:402–407
Smeacetto F, Chrysanthou A, Salvo M et al (2011) Thermal cycling and ageing of a glass-ceramic sealant for planar SOFCs. Int J Hydrogen Energy 36:11895–11903
Smeacetto F, Salvo M, Leone P et al (2011) Performance and testing of joined Crofer22APU-glass-ceramic sealant-anode supported cell in SOFC relevant conditions. Mater Lett 65:1048–1052
Smeacetto F, Salvo M, Santarelli M, Leone P et al (2013) Performance of a glass-ceramic sealant in a SOFC short stack. Int J Hydrogen Energy 38:588–596
Smeacetto F, Chrysanthou A, Moskalewicz T, Salvo M (2013) Thermal cycling of Crofer22APU-sealant-anode supported electrolyte joined structures for planar SOFCs up to 3000 h. Mater Lett 111:143–146
Smeacetto F, Radaelli M, Salvo M et al (2017) Glass-ceramic joining material for sodium-based battery. J Ceram Int 43:8329–8333
Smeacetto F, Miranda A, Polo SC et al (2015) Electrophoretic deposition of Mn1.5Co1.5O4 on metallic interconnect and interaction with glass-ceramic sealant for solid oxide fuel cells application. J Power Sources 280:379–386
Smeacetto F, De Miranda A, Ventrella A, Salvo M, Ferraris M (2015) Shear strength tests of glass ceramic sealant for solid oxide fuel cells applications. Adv Appl Ceram 114:70–75
Smeacetto F, Salvo M, D’Hérin Bytner FD, Leone P, Ferraris M (2010) New glass and glass-ceramic sealants for planar solid oxide fuel cells. J Eur Chem Soc 30:933–940
Sabato AG, Cempura G, Montinaro D, Chrysanthou A, Salvo M, Bernardo E, Secco M, Smeacetto F (2016) Glass-ceramic sealant for solid oxide fuel cells application: characterization and performance in dual atmosphere. J Power Sources 328:262–270
Sabato AG, Salvo M, De Miranda A, Smeacetto F (2015) Crystallization behaviour of glass-ceramic sealant for solid oxide fuel cells. Mater Lett 141:284–287
Krainova DA, Zharkinova ST, Saetova NS et al (2017) Influence of cerium oxide on properties of glass-ceramic sealants for solid oxide fuel cells. Rus J Appl Chem 90:1047–1053
Gropp S, Fischer M, Dittrich L et al (2015) Wetting behaviour of glasses on nanostructured silicon surfaces. J Electr Eng 3:15–20
Borhan AI, Gromada M, Nedelcu GG, Leontie L (2016) Influence of (CoO, CaO, B2O3) additives on thermal and dielectric properties of BaO–Al2O3–SiO2 glass ceramic sealant for OTM applications. J Ceram Int 42:10459–10468
Pershina SV, Raskovalov AA, Antonov BD et al (2015) Extreme behavior of Li-ion conductivity in the Li2O–Al2O3–P2O5 glass system. J Non-Cryst Solids 430:64–72
Nechaev GV, Vlasova SG, Reznitskikh OG (2015) Conductivity in sodium-yttrium-silicate and sodium-yttrium-phosphate glass. Glass Phys Chem 41:64–67
Lasocka M (1976) The effect of scanning rate on glass transition temperature of splat-cooled Te85Ge15. J Mater Sci Eng 23:173–177
Ota T, Matsubara T, Takahashi M et al (1995) Thermal expansion of nepheline–leucite ceramic composites. J Ceram Soc Jpn 103:523–524
Appen AA (1974) Himiya stekla. Himia, Leningrad
Fluegel Alexander (2010) Thermal expansion calculation for silicate glasses at 210°C based on a systematic analysis of global databases. Glass Technol-Part A 51:191–201
Chou Y, Stevenson JW, Singh P (2008) Effect of aluminizing of Cr-containing ferritic alloys on the seal strength of a novel high-temperature solid oxide fuel cell sealing glass. J Power Sources 185:1001–1008
Reis ST, Pascual MJ, Brow RK et al (2010) Crystallization and processing of SOFC sealing glasses. J Non-Cryst Solids 356:3009–3012
Lee YM, Nassaralla CL (2001) Heat capacities of calcium chromate and calcium chromite. Thermochim Acta 371:1–5
Glushko VP, Gurvich LV, Bergman GA, Veits IV, Medvedev VA, Khachkuruzov GA, Yungman VS (1978–1982) Thermodynamic properties of individual materials, Nauka, Moskva (Russia), V. I–IV
Schmetterer C, Masset PJ (2012) Heat capacity of compounds in the CaO–SiO2 system—a review. J Phase Equilibria Diffusion 33:261–275
Nisino T, Moteki K, Sikano H (1963) Thermal decomposition of magnesium chromate. J Ceram Assoc 71:159–163
Acknowledgements
This study was financially supported by the RFBR-BRFFR project (RFBR Grant No. 17-58-04116, BRFFR Grant No. X17PM-033). The research was partially performed using the facilities of the Shared Access Centre “Composition of Compounds” of IHTE UB RAS. The authors thank S.V. Plaksin, E.A. Sherstobitova and V.A. Eremin for their assistance in conducting the research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saetova, N.S., Krainova, D.A., Kuzmin, A.V. et al. Alumina–silica glass–ceramic sealants for tubular solid oxide fuel cells. J Mater Sci 54, 4532–4545 (2019). https://doi.org/10.1007/s10853-018-3181-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-018-3181-8