Abstract
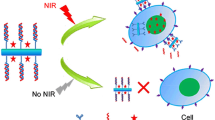
Photothermal therapy is a highly efficient and minimally invasive method for cancer therapy. To enhance the safe and effective effect of photothermal therapy, specific recognition of targeting cells and efficient direct heat transmission to cells for killing cells are quite important. In this work, aptamer-conjugated gold nanostars (Apt-AuNSs) used for targeted photothermal therapy were reported. AuNSs showed a higher photothermal conversion efficiency and excellent photostability, which has been used as a highly effective theranostic nanoprobe. Thiol-modified AS1411 aptamers, which showed the high targeting property for HeLa cells with overexpression of nucleolin, were conjugated to AuNSs through Au–S bond. In vitro toxicity assessments illustrated that Apt-AuNSs have low cytotoxicity and suitable for biological applications. Furthermore, the accumulation of Apt-AuNSs in HeLa cells was observed via TEM image. The targeted photothermal therapy in vitro and apoptosis assay results showed excellent anticancer effect. These results suggest that the targeted Apt-AuNSs exhibit great potential in selective photothermal therapy of cancer.









Similar content being viewed by others
References
Chen Z, Yu D, Huang Y, Zhang Z, Liu T, Zhan J (2014) Tunable SERS-tags-hidden gold nanorattles for theranosis of cancer cells with single laser beam. Sci Rep 4:6709
Liu SY, Liang ZS, Gao F, Luo SF, Lu GQ (2010) In vitro photothermal study of gold nanoshells functionalized with small targeting peptides to liver cancer cells. J Mater Sci Mater 21:665–674
Cheng M, Wang H, Zhang Z, Li N, Fang X, Xu S (2014) Gold nanorod-embedded electrospun fibrous membrane as a photothermal therapy platform. ACS Appl Mater Inter 6:1569–1575
Huang Z, Qi Y, Yu D, Zhan J (2016) Radar-like MoS2 nanoparticles as a high efficient 808 nm laser-induced photothermal agent for cancer therapy. RSC Adv 6:31031–31036
Kam NWS, O’Connell M, Wisdom JA, Dai H (2005) Carbon nanotubes as multifunctional biological transporters and near-infrared agents for selective cancer cell destruction. Proc Natl Acad Sci USA 102:11600–11605
Lal S, Clare SE, Halas NJ (2009) Nanoshell-enabled photothermal cancer therapy: impending clinical impact. Cheminform 40:1842–1851
Jang B, Park JY, Tung CH, Kim IH, Choi Y (2011) Gold nanorod-photosensitizer complex for near-infrared fluorescence imaging and photodynamic/photothermal therapy in vivo. ACS Nano 5:1086–1094
Cheng L, Yang K, Li Y, Chen J, Wang C, Shao M, Lee ST, Liu Z (2011) Facile preparation of multifunctional upconversion nanoprobes for multimodal imaging and dual-targeted photothermal therapy. Angew Chem Int Ed Engl 123:7523–7528
Yang L, Tseng YT, Suo G, Chen L, Yu J, Chiu WJ, Huang CC, Lin CH (2015) Photothermal therapeutic response of cancer cells to aptamer-gold nanoparticle-hybridized graphene oxide under NIR illumination. ACS Appl Mater Inter 7:5097–5106
Yang X, Yang M, Pang B, Vara M, Xia Y (2015) Gold nanomaterials at work in biomedicine. Chem Rev 115:10410–10488
Zhang J, Yuan ZF, Wang Y, Chen WH, Luo GF, Cheng SX, Zhuo RX, Zhang XZ (2013) Multifunctional envelope-type mesoporous silica nanoparticles for tumor-triggered targeting drug delivery. J Am Chem Soc 135:5068–5073
Noh MS, Lee S, Kang H, Yang JK, Lee H, Hwang D, Lee JW, Jeong S, Jang Y, Jun BH (2015) Target-specific near-IR induced drug release and photothermal therapy with accumulated Au/Ag hollow nanoshells on pulmonary cancer cell membranes. Biomaterials 45:81–92
Shi J, Wang L, Zhang J, Ma R, Gao J, Liu Y, Zhang C, Zhang Z (2014) A tumor-targeting near-infrared laser-triggered drug delivery system based on GO@Ag nanoparticles for chemo-photothermal therapy and X-ray imaging. Biomaterials 35:5847–5861
Li W, Rong P, Yang K, Huang P, Sun K, Chen X (2015) Semimetal nanomaterials of antimony as highly efficient agent for photoacoustic imaging and photothermal therapy. Biomaterials 45:18–26
Hu KW, Liu TM, Chung K, Huang KS, Hsieh CT, Sun CK, Yeh CS (2009) Efficient near-IR hyperthermia and intense nonlinear optical imaging contrast on the gold nanorod-in-shell nanostructures. J Am Chem Soc 131:14186–14187
Huang X, Elsayed IH, Qian W, Elsayed MA (2006) Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J Am Chem Soc 128:2115–2120
Liu H, Chen D, LiL Liu T, Tan L, Wu X, Tang F (2011) Multifunctional gold nanoshells on silica nanorattles: a platform for the combination of photothermal therapy and chemotherapy with low systemic toxicity. Angew Chem Int Ed Engl 50:891–895
Zhang AW, Guo WH, Qi YF, Wang JZ, Ma XX, Yu DX (2016) Synergistic effects of gold nanocages in hyperthermia and radiotherapy treatment. Nanoscale Res Lett 11:1–14
Tian Y, Luo S, Yan H, Teng Z, Pan Y, Zeng L, Wu J, Li Y, Liu Y, Wang S (2015) Gold nanostars functionalized with amine-terminated PEG for X-ray/CT imaging and photothermal therapy. J Mater Chem B 3:4330–4337
Chen H, Liu F, Lei Z, Ma L, Wang Z (2015) Fe2O3@Au core@shell nanoparticle–graphene nanocomposites as theranostic agents for bioimaging and chemo-photothermal synergistic therapy. RSC Adv 5:84980–84987
Curry T, Kopelman R, Shilo M, Popovtzer R (2014) Multifunctional theranostic gold nanoparticles for targeted CT imaging and photothermal therapy. Contrast Media Mol Imaging 9:53–61
D’Hollander A, Mathieu E, Jans H, Velde GV, Stakenborg T, Dorpe PV, Himmelreich U, Lagae L (2016) Development of nanostars as a biocompatible tumor contrast agent: toward in vivo SERS imaging. Int J Nanomed 11:3703–3714
Yuan H, Khoury CG, Hwang H, Wilson CM, Grant GA, Vodinh T (2012) Gold nanostars: surfactant-free synthesis, 3D modelling, and two-photon photoluminescence imaging. Nanotechnology 23:075102
Liu Y, Zhi X, Yang M, Zhang J, Lin L, Zhao X, Hou W, Zhang C, Zhang Q, Pan F (2017) Tumor-triggered drug release from calcium carbonate-encapsulated gold nanostars for near-infrared photodynamic/photothermal combination antitumor therapy. Theranostics 7:1650–1662
Qin XC, Guo ZY, Liu ZM, Zhang W, Wan MM, Yang BW (2013) Folic acid-conjugated graphene oxide for cancer targeted chemo-photothermal therapy. J Photochem Photobiol B 120:156–162
Bocafarcau S, Potara M, Simon T, Juhem A, Baldeck P, Astilean S (2014) Folic acid-conjugated, SERS-labeled silver nanotriangles for multimodal detection and targeted photothermal treatment on human ovarian cancer cells. Mol Pharm 11:391–399
Yuan H, Fales AM, Vodinh T (2012) TAT peptide-functionalized gold nanostars: enhanced intracellular delivery and efficient NIR photothermal therapy using ultralow irradiance. J Am Chem Soc 134:11358–11361
Dam DHM, Lee JH, Sisco PN, Co DT, Zhang M, Wasielewski MR, Odom TW (2012) Direct observation of nanoparticle-cancer cell nucleus interactions. ACS Nano 6:3318–3326
Liang H, Zhang XB, Lv Y, Gong L, Wang R, Zhu X, Yang R, Tan W (2012) Functional DNA-containing nanomaterials: cellular applications in biosensing, imaging, and targeted therapy. Acc Chem Res 47:1891–1901
Huang YF, Sefah K, Bamrungsap S, Chang HT, Tan W (2008) Selective photothermal therapy for mixed cancer cells using aptamer-conjugated nanorods. Langmuir 24:11860–11865
Wang J, Sefah K, Altman MB, Chen T, You M, Zhao Z, Huang CZ, Tan W (2013) Aptamer-conjugated nanorods for targeted photothermal therapy of prostate cancer stem cells. Chem Asian J 8:2417–2422
Xiao Z, Farokhzad OC (2012) Aptamer-functionalized nanoparticles for medical applications: challenges and opportunities. ACS Nano 6:3670–3676
Liu Y, Ashton JR, Moding EJ, Yuan H, Register JK, Fales AM, Choi J, Whitley MJ, Zhao X, Qi Y (2015) A plasmonic gold nanostar theranostic probe for in vivo tumor imaging and photothermal therapy. Theranostics 5:946–960
Wang S, Teng Z, Huang P, Liu D, Liu Y, Tian Y, Sun J, Li Y, Ju H, Chen X (2015) Reversibly extracellular pH controlled cellular uptake and photothermal therapy by PEGylated mixed-charge gold nanostars. Small 11:1801–1810
Liang S, Li C, Zhang C, Chen Y, Xu L, Bao C, Wang X, Liu G, Zhang F, Cui D (2015) CD44v6 monoclonal antibody-conjugated gold nanostars for targeted photoacoustic imaging and plasmonic photothermal therapy of gastric cancer stem-like cells. Theranostics 5:970–984
Dam DHM, Lee JH, Sisco PN, Co DT, Zhang M, Wasielewski MR, Odom TW (2015) Direct observation of nanoparticle-cancer cell nucleus interactions. ACS Nano 6:3318–3326
Wu P, Gao Y, Zhang H, Cai C (2012) Aptamer-guided silver-gold bimetallic nanostructures with highly active surface-enhanced raman scattering for specific detection and near-infrared photothermal therapy of human breast cancer cells. Anal Chem 84:7692–7699
Wu P, Gao Y, Lu Y, Zhang H, Cai C (2013) High specific detection and near-infrared photothermal therapy of lung cancer cells with high SERS active aptamer-silver–gold shell–core nanostructures. Analyst 138:6501–6510
Dam DHM, Lee RC, Odom TW (2014) Improved in vitro efficacy of gold nanoconstructs by increased loading of G-quadruplex aptamer. Nano Lett 14:2843–2848
Yuan H, Liu Y, Fales AM, Li YL, Liu J, Vo-Dinh T (2013) Quantitative surface-enhanced resonant Raman scattering multiplexing of biocompatible gold nanostars for in vitro and ex vivo detection. Anal Chem 85:208–212
Abbaspour A, Norouzsarvestani F, Noori A, Soltani N (2015) Aptamer-conjugated silver nanoparticles for electrochemical dual-aptamer-based sandwich detection of staphylococcus aureus. Biosens Bioelectron 68:149–155
Manivasagan P, Bharathiraja S, Moorthy MS, Oh YO, Song K, Seo H, Oh J (2017) Anti-EGFR antibody conjugation of fucoidan-coated gold nanorods as novel photothermal ablation agents for cancer therapy. ACS Appl Mater Inter 9:14633–14646
Wu X, Zhou L, Su Y, Dong CM (2016) Plasmonic, targeted, and dual drugs-loaded polypeptide composite nanoparticles for synergistic cocktail chemotherapy with photothermal therapy. Biomacromol 17:2489–2501
Acknowledgements
We are grateful for financial support from National Natural Science Foundation of China (NSFC81602736), Shandong Provincial Natural Science Foundation (ZR2015HQ014, ZR2016HB73, and ZR2017LH050), China Postdoctoral Science Foundation (2018M632684, 2015M580595), the Medical and Health Science and Technology Development Projects of Shandong Province (2015WS0382, 2016WS0215, and 2016WS0210), and Special Funds for Postdoctoral Innovative Projects of Shandong Province.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, Y., Wang, X., Gao, L. et al. Aptamer-conjugated gold nanostars for targeted cancer photothermal therapy. J Mater Sci 53, 14138–14148 (2018). https://doi.org/10.1007/s10853-018-2668-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-018-2668-7