Abstract
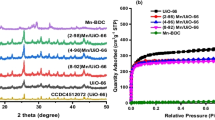
In the present paper, the microwave synthesis of MOF-199 and its application as an electrode modifier for the simultaneous voltammetric determination of paracetamol (PAR) and caffeine (CAF) were demonstrated. The obtained materials were characterised by X-ray diffraction, a scanning electron microscope (SEM), nitrogen adsorption/desorption isotherms and thermal gravity. The microwave (MW) synthesis of MOF-199 has been compared to its conventional hydrothermal synthesis. It is found that by using the MW synthesis, MOF-199 can be obtained in a much shorter synthesis time with improved yield and textural properties. The electrode modified by MOF-199 was used in order to develop an electroanalytical method that can be used to simultaneously quantify PAR and CAF. The kinetic parameters of the electrode reaction process were also investigated. This proposed method was successfully employed for the simultaneous detection of PAR and CAF in pharmaceutical formulations using the standard addition method and the obtained results compared with the results determined by means of HPLC were found to be statistically similar.














Similar content being viewed by others
References
Kachoosangi RT, Wildgoose GG, Compton RG (2008) Sensitive adsorptive stripping voltammetric determination of paracetamol at multiwalled carbon nanotube modified basal plane pyrolytic graphite electrode. Anal Chim Acta 618:54–60
Rostagno M, Manchón N, D’Arrigo M, Guillamón E, Villares A, García-Lafuente A, Ramos A, Martínez J (2011) Fast and simultaneous determination of phenolic compounds and caffeine in teas, mate, instant coffee, soft drink and energetic drink by high-performance liquid chromatography using a fused-core column. Anal Chim Acta 685:204–211
Zen J-M, Ting Y-S, Shih Y (1998) Voltammetric determination of caffeine in beverages using a chemically modified electrode. Analyst 123:1145–1147
Reynolds JEF (1996) Martindale: the extra pharmacopoeia. Pharmaceutical Press, London
The United States Pharmacopeia (2000) U.S. Pharmacopeial Convention, Rockville
Sirajuddin AR, Khaskheli AR, Shah A, Bhanger MI, Niaz A, Mahesar S (2007) Simpler spectrophotometric assay of paracetamol in tablets and urine samples. Spectrochim Acta Part A Mol Biomol Spectrosc 68:747–751
Chiou J-F, Chen S-L, Chen S-M, Tsou S-S (2008) Novel spectrophotometric method for RAPID quantifying acetaminophen concentration in emergent situation. J Food Drug Anal 16:36–40
Kartal M (2001) LC method for the analysis of paracetamol, caffeine and codeine phosphate in pharmaceutical preparations. J Pharm Biomed Anal 26:857–864
Gómez MJ, Petrović M, Fernández-Alba AR, Barceló D (2006) Determination of pharmaceuticals of various therapeutic classes by solid-phase extraction and liquid chromatography–tandem mass spectrometry analysis in hospital effluent wastewaters. J Chromatogr A 1114:224–233
Moţ AC, Soponar F, Medvedovici A, Sârbu C (2010) Simultaneous spectrophotometric determination of aspirin, paracetamol, caffeine, and chlorphenamine from pharmaceutical formulations using multivariate regression methods. Anal Lett 43:804–813
Llorent-Martínez E, Šatínský D, Solich P, Ortega-Barrales P, Molina-Díaz A (2007) Fluorimetric SIA optosensing in pharmaceutical analysis: determination of paracetamol. J Pharm Biomed Anal 45:318–321
Dejaegher B, Bloomfield M, Smeyers-Verbeke J, Vander Heyden Y (2008) Validation of a fluorimetric assay for 4-aminophenol in paracetamol formulations. Talanta 75:258–265
Dou Y, Sun Y, Ren Y, Ju P, Ren Y (2005) Simultaneous non-destructive determination of two components of combined paracetamol and amantadine hydrochloride in tablets and powder by NIR spectroscopy and artificial neural networks. J Pharm Biomed Anal 37:543–549
Baptistao M, de Carvalho Rocha WF, Poppi RJ (2011) Quality control of the paracetamol drug by chemometrics and imaging spectroscopy in the near infrared region. J Mol Struct 1002:167–171
Lourencao BC, Medeiros RA, Rocha-Filho RC, Mazo LH, Fatibello-Filho O (2009) Simultaneous voltammetric determination of paracetamol and caffeine in pharmaceutical formulations using a boron-doped diamond electrode. Talanta 78:748–752
Noviandri I, Rakhmana R (2012) Carbon paste electrode modified with carbon nanotubes and poly (3-aminophenol) for voltammetric determination of paracetamol. Int J Electrochem Sci 7:4479–4487
Bayram E, Akyilmaz E (2016) Development of a new microbial biosensor based on conductive polymer/multiwalled carbon nanotube and its application to paracetamol determination. Sens Actuators B Chem 233:409–418
Phan A, Doonan CJ, Uribe-Romo FJ, Knobler CB, O’keeffe M, Yaghi OM (2010) Synthesis, structure, and carbon dioxide capture properties of zeolitic imidazolate frameworks. Acc Chem Res 43:58–67
Xiao L, Xu H, Zhou S, Song T, Wang H, Li S, Gan W, Yuan Q (2014) Simultaneous detection of Cd(II) and Pb(II) by differential pulse anodic stripping voltammetry at a nitrogen-doped microporous carbon/Nafion/bismuth-film electrode. Electrochim Acta 143:143–151
Zen J-M, Ting Y-S (1997) Simultaneous determination of caffeine and acetaminophen in drug formulations by square-wave voltammetry using a chemically modified electrode. Anal Chim Acta 342:175–180
Sanghavi BJ, Srivastava AK (2010) Simultaneous voltammetric determination of acetaminophen, aspirin and caffeine using an in situ surfactant-modified multiwalled carbon nanotube paste electrode. Electrochim Acta 55:8638–8648
Wang S-F, Xie F, Hu R-F (2007) Carbon-coated nickel magnetic nanoparticles modified electrodes as a sensor for determination of acetaminophen. Sens Actuators B Chem 123:495–500
Salehi S, Anbia M (2017) High CO2 adsorption capacity and CO2/CH4 selectivity by nanocomposites of MOF-199. Energy Fuels 31:5376–5384
Ho SL, Yoon IC, Cho CS, Choi H-J (2015) A recyclable metal-organic framework MOF-199 catalyst in coupling and cyclization of β-bromo-α, β-unsaturated carboxylic acids with terminal alkynes leading to alkylidenefuranones. J Organomet Chem 791:13–17
Pohle R, Tawil A, Davydovskaya P, Fleischer M (2011) Metal organic frameworks as promising high surface area material for work function gas sensors. Proc Eng 25:108–111
Loera-Serna S, Oliver-Tolentino MA, de Lourdes López-Núñez M, Santana-Cruz A, Guzmán-Vargas A, Cabrera-Sierra R, Beltrán HI, Flores J (2012) Electrochemical behavior of [Cu3(BTC)2] metal–organic framework: the effect of the method of synthesis. J Alloys Compd 540:113–120
Khan IA, Badshah A, Nadeem MA, Haider N, Nadeem MA (2014) A copper based metal-organic framework as single source for the synthesis of electrode materials for high-performance supercapacitors and glucose sensing applications. Int J Hydrog Energy 39:19609–19620
Zhou J, Li X, Yang L, Yan S, Wang M, Cheng D, Chen Q, Dong Y, Liu P, Cai W (2015) The Cu-MOF-199/single-walled carbon nanotubes modified electrode for simultaneous determination of hydroquinone and catechol with extended linear ranges and lower detection limits. Anal Chim Acta 899:57–65
Wang X, Lu X, Wu L, Chen J (2015) 3D metal-organic framework as highly efficient biosensing platform for ultrasensitive and rapid detection of bisphenol A. Biosens Bioelectron 65:295–301
Chui SS-Y, Lo SM-F, Charmant JPH, Orpen AG, Williams ID (1999) A chemically functionalizable nanoporous material [Cu3(TMA)2(H2O)3]n. Science 283:1148–1150
Vishnyakov A, Ravikovitch PI, Neimark AV, Bülow M, Wang QM (2003) Nanopore structure and sorption properties of Cu-BTC metal-organic framework. Nano Lett 3:713–718
Seo Y-K, Hundal G, Jang IT, Hwang YK, Jun C-H, Chang J-S (2009) Microwave synthesis of hybrid inorganic–organic materials including porous Cu3(BTC)2 from Cu(II)-trimesate mixture. Microporous Mesoporous Mater 119:331–337
Mueller U, Schubert M, Teich F, Puetter H, Schierle-Arndt K, Pastre J (2006) Metal–organic frameworks—prospective industrial applications. J Mater Chem 16:626–636
Li Z-Q, Qiu L-G, Xu T, Wu Y, Wang W, Wu Z-Y, Jiang X (2009) Ultrasonic synthesis of the microporous metal–organic framework Cu3(BTC)2 at ambient temperature and pressure: an efficient and environmentally friendly method. Mater Lett 63:78–80
Qiu L-G, Li Z-Q, Wu Y, Wang W, Xu T, Jiang X (2008) Facile synthesis of nanocrystals of a microporous metal–organic framework by an ultrasonic method and selective sensing of organoamines. Chem Commun 31:3642–3644
Haque E, Khan NA, Park JH, Jhung SH (2010) Synthesis of a metal–organic framework material, iron terephthalate, by ultrasound, microwave, and conventional electric heating: a kinetic study. Chem A Eur J 16:1046–1052
Ni Z, Masel RI (2006) Rapid production of metal–organic frameworks via microwave-assisted solvothermal synthesis. J Am Chem Soc 128:12394–12395
Jhung SH, Lee JH, Forster PM, Férey G, Cheetham AK, Chang JS (2006) Microwave synthesis of hybrid inorganic–organic porous materials: phase-selective and rapid crystallization. Chem A Eur J 12:7899–7905
Jhung SH, Lee JH, Yoon JW, Serre C, Férey G, Chang JS (2007) Microwave synthesis of chromium terephthalate MIL-101 and its benzene sorption ability. Adv Mater 19:121–124
Klinowski J, Paz FA, Silva P, Rocha J (2011) Microwave-assisted synthesis of metal-organic frameworks. Dalton Trans 40:321–330
Tompsett GA, Conner WC, Yngvesson KS (2006) Microwave synthesis of nanoporous materials. ChemPhysChem 7:296–319
Schlichte K, Kratzke T, Kaskel S (2004) Improved synthesis, thermal stability and catalytic properties of the metal–organic framework compound Cu3(BTC)2. Microporous Mesoporous Mater 73:81–88
Marx S, Kleist W, Baiker A (2011) Synthesis, structural properties, and catalytic behavior of Cu-BTC and mixed-linker Cu-BTC-PyDC in the oxidation of benzene derivatives. J Catal 281:76–87
Alaerts L, Seguin E, Poelman H, Thibault-Starzyk F, Jacobs PA, De Vos DE (2006) Probing the Lewis acidity and catalytic activity of the metal-organic framework [Cu3(BTC)2] (BTC = benzene-1,3,5-tricarboxylate). Chemistry 12:7353–7363
Krawiec P, Kramer M, Sabo M, Kunschke R, Fröde H, Kaskel S (2006) Improved hydrogen storage in the metal-organic framework Cu3(BTC)2. Adv Eng Mater 8:293–296
Miner DJ, Rice JR, Riggin RM, Kissinger PT (1981) Voltammetry of acetaminophen and its metabolites. Anal Chem 53:2258–2263
Chitravathi S, Munichandraiah N (2016) Voltammetric determination of paracetamol, tramadol and caffeine using poly (Nile blue) modified glassy carbon electrode. J Electroanal Chem 764:93–103
Soleymani J, Hasanzadeh M, Shadjou N, Jafari MK, Gharamaleki JV, Yadollahi M, Jouyban A (2016) A new kinetic–mechanistic approach to elucidate electrooxidation of doxorubicin hydrochloride in unprocessed human fluids using magnetic graphene based nanocomposite modified glassy carbon electrode. Mater Sci Eng C 61:638–650
Bard AJ, Faulkner LR (2001) Fundamentals and applications: electrochemical methods. Wiley, New York
Laviron E (1979) General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J Electroanal Chem Interfacial Electrochem 101:19–28
Li C (2007) Electrochemical determination of dipyridamole at a carbon paste electrode using cetyltrimethyl ammonium bromide as enhancing element. Colloids Surf B 55:77–83
Švorc Lu, Tomčík P, Svítková J, Rievaj M, Bustin D (2012) Voltammetric determination of caffeine in beverage samples on bare boron-doped diamond electrode. Food Chem 135:1198–1204
Sharp M, Petersson M, Edström K (1979) Preliminary determinations of electron transfer kinetics involving ferrocene covalently attached to a platinum surface. J Electroanal Chem Interfacial Electrochem 95:123–130
Horwitz W, Albert R (1997) Quality issues the concept of uncertainty as applied to chemical measurements. Analyst 122:615–617
Zhang X, Wu L, Zhou J, Zhang X, Chen J (2015) A new ratiometric electrochemical sensor for sensitive detection of bisphenol A based on poly-β-cyclodextrin/electroreduced graphene modified glassy carbon electrode. J Electroanal Chem 742:97–103
Habibi B, Gahramanzadeh R (2011) Fabrication and characterization of non-platinum electrocatalyst for methanol oxidation in alkaline medium: nickel nanoparticles modified carbon-ceramic electrode. Int J Hydrog Energy 36:1913–1923
Lau O-W, Luk S-F, Cheung Y-M (1989) Simultaneous determination of ascorbic acid, caffeine and paracetamol in drug formulations by differential-pulse voltammetry using a glassy carbon electrode. Analyst 114:1047–1051
Amiri-Aref M, Raoof JB, Ojani R (2014) A highly sensitive electrochemical sensor for simultaneous voltammetric determination of noradrenaline, acetaminophen, xanthine and caffeine based on a flavonoid nanostructured modified glassy carbon electrode. Sens Actuators B Chem 192:634–641
Saciloto TR, Cervini P, Cavalheiro ÉT (2013) Simultaneous voltammetric determination of acetaminophen and caffeine at a graphite and polyurethane screen-printed composite electrode. J Braz Chem Soc 24:1461–1468
Eisele APP, Clausen DN, Tarley CRT, Dall’Antonia LH, Sartori ER (2013) Simultaneous square-wave voltammetric determination of paracetamol, caffeine and orphenadrine in pharmaceutical formulations using a cathodically pretreated boron-doped diamond electrode. Electroanalysis 25:1734–1741
Acknowledgements
This work was supported by the project B2016-DHH-20 sponsored by Ministry of Education and Training, Vietnam.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Minh, T.T., Phong, N.H., Van Duc, H. et al. Microwave synthesis and voltammetric simultaneous determination of paracetamol and caffeine using an MOF-199-based electrode. J Mater Sci 53, 2453–2471 (2018). https://doi.org/10.1007/s10853-017-1715-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-017-1715-0