Abstract
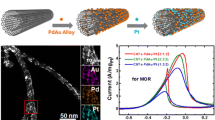
Pt-decorated \(\hbox {TiO}_{2}\) nanotubes Pt@TiO2 are prepared only by applying a set of facile wet-chemical redox reactions to ion track-etched polycarbonate templates. First, a homogeneous layer of Pt nanoparticles is deposited onto the complex template surface by reducing potassium tetrachloroplatinate with absorbed dimethylaminoborane. Second, the template is coated with a conformal \(\hbox {TiO}_{2}\) layer, using a chemical bath deposition reaction based on titanium(III) chloride. After the removal of the template, the rutile-type \(\hbox {TiO}_{2}\) nanotubes remain decorated with Pt nanoparticles and nanoparticle-clusters on their outside. During the process, neither vacuum techniques nor external current sources or addition of heat are employed. The crystallinity, composition, and morphology of the composite nanotubes are analysed by X-ray diffraction, scanning and transmission electron microscopy as well as by energy-dispersive X-ray spectroscopy. Finally, the obtained materials are examplarily applied in the electrooxidation of ethanol and formic acid, and their performances have been evaluated. Compared to conventional carbon black-supported Pt nanoparticles, the Pt@TiO2 nanotubes show higher reaction rates. Mass activities of 2.36 \(\hbox {A}\hbox { mg}_{\rm Pt}^{-1}\hbox { cm}^{-2}\) are reached in ethanol oxidation and 7.56 \(\hbox {A}\hbox { mg}_{\rm Pt}^{-1}\hbox { cm}^{-2}\) in the formic acid oxidation. The present structures are able to exploit the synergy of Pt and \(\hbox {TiO}_{2}\) with a bifunctional mechanism to result in powerful but easy-to-fabricate catalyst structures. They represent an easily producible type of composite nanostructures which can be applied in various fields such as in catalytics and sensor technology.








Similar content being viewed by others
References
Huang X-J, Choi Y-K (2007) Chemical sensors based on nanostructured materials. Sens Actuators B 122:659–671
Barsan N, Koziej D, Weimar U (2007) Metal oxide-based gas sensor research: how to? Sens Actuators B 121:18–35
Zhu C, Yang G, Li H, Dan D, Lin Y (2015) Electrochemical sensors and biosensors based on nanomaterials and nanostructures. Anal Chem 87:230–249
Lucia U (2014) Overview on fuel cells. Renew Sustain Energy Rev 30:164–169
Sharaf OZ, Orhan MF (2014) An overview of fuel cell technology: fundamentals and applications. Renew Sustain Energy Rev 32:810–853
Qiu J-D, Wang G-C, Liang R-P, Xia X-H, Hong-Wen Y (2011) Controllable deposition of platinum nanoparticles on graphene as and electrocatalyst for direct methanol fuel cells. J Phys Chem C 115:15639–15645
Zhang C, Hongmei Y, Li F, Xiao Y, Gao Y, Li Y, Zeng Y, Jia J, Yi B, Shao Z (2015) An oriented ultrathin catalyst layer derived from high conductive TiO\(_{2}\) nanotube for polymer electrolyte membrane fuel cell. Electrochim Acta 153:361–369
Clark JH (2016) Green and sustainable chemistry: an introduction. In: Green and sustainable medicinal chemistry: methods, tools and strategies for the 21st century pharameceutical industry, number 46 in RSC Green Chemistry. The Royal Society of Chemistry, Cambridge
Chow J, Kopp RJ, Portney PR (2003) Energy resources and global development. Science 302(5650):1528–1531
Shafiee S, Topal E (2009) When will fossil fuel reserves be diminished? Energy Policy 37:181–189
Pagliaro M, Konstandopoulos AG, Ciriminna R, Palmisano G (2010) Solar hydrogen: fuel of the near future. Energy Environ Sci 3:279–287
Zhang S, Shao Y, Yin G, Lin Y (2013) Recent progress in nanostructured electrocatalyst PEM fuel cells. J Mater Chem A 1:4631–4641
An L, Chen R (2016) Direct formate fuel cells: a review. J Power Sources 320:127–139
Changwei X, Shen P, Liu Y (2007) Ethanol electrooxidation on Pt/C and Pd/C catalysts promoted with oxide. J Power Sources 164:527–531
Yan Qiao and Chang Ming Li (2011) Nanostructured catalysts in fuel cells. J Mater Chem 21:4027–4036
Rasmi KR, Vanithakumari SC, George RP, Mallika C, Kamachi Mudali U (2015) Nanoparticles of Pt loaded on a vertically aligned TiO\(_{2}\) nanotube bed. RSC Adv 5:108050–108057
Zhou W, Zhou Z, Song S, Li W, Sun G, Tsiakaras P, Xin Q (2003) Pt based anode catalysts for direct ethanol fuel cells. Appl Catal B 46:273–285
Beyhan S, Coutanceau C, Léger J-M, Napporn TW, Kadirgan F (2013) Promising anode candidates for direct ethanol fuel cell: carbon supported PtSn-based trimetallic catalysts prepared by Bönnemann method. Int J Hydrogen Energy 38:6830–6841
Tayal J, Rawat B, Basu S (2012) Effect of addition of rhenium to Pt-based anode catalysts in electro-oxidation of ethanol in direct ethanol PEM fuel cell. Int J Hydrogen Energy 37:4597–4605
Akhairi MAF, Kamarudin SK (2016) Catalysts in direct ethanol fuel cell (DEFC): an overview. Int J Hydrogen Energy 41:4214–4226
Hou J, Shao Y, Ellis MW, Moore RB, Yi B (2011) Graphene-based electrochemical energy conversion and storage: fuel cells, supercapacitors and lithium ion batteries. Phys Chem Chem Phys 13:15384–15402
Han M, Li M, Xin W, Zeng J, Liao S (2015) Highly stable and active Pt electrocatalyst on TiO\(_{2}\)-CoO4-C composite support for polymer exchange membrane fuel cells. Electrochim Acta 154:266–272
Sui X-L, Wang Z-B, Li C-Z, Zhang J-J, Zhao L, Da-Ming G (2014) Effect of pH value on H\(_{2}\)Ti\(_{2}\)O\(_{5}\)/TiO\(_{2}\) composite nanotubes as pt catalyst support for methanol oxidation. J Power Sources 272:196–202
Lei D, Shao Y, Sun J, Yin G, Liu J, Wang Y (2016) Advanced catalyst supports for PEM fuel cell cathodes. Nano Energy 29:314–322
Eberle U, Müller B, von Helmolt R (2012) Fuel cell electric vehicles and hydrogen infrastructure: status 2012. Energy Environ Sci 5:8780–8798
Zhao L, Wang Z-B, Liu J, Zhang J-J, Sui X-L, Zhang L-M, Da-Ming G (2015) Facile one-pot synthesis of Pt/graphene-TiO\(_{2}\) hybrid catalyst with enhanced methanol electrooxidation performance. J Power Sources 279:210–217
Liu J, Liu B, Ni Z, Deng Y, Zhong C, Wenbin H (2014) Improved catalytic performance of Pt/TiO\(_{2}\) nanotubes electrode for ammonia oxidation under UV-light illumination. Electrochim Acta 150:146–150
Liu R, Sen A (2012) Controlled synthesis of heterogeneous metal–titania nanostructures and their applications. J Am Chem Soc 134(42):17505–17512
Boehme M, Ensinger W (2011) Mixed phase anatase/rutile titanium dioxide nanotubes for enhanced photoctalytic degradation of methylene-blue. Nano-Micro Lett 3(4):236–241
Qiao P, Zou S, Shaodan X, Liu J, Li Y, Ma G, Xiao L, Lou H, Fan J (2014) A general synthesis strategy of multi-metallic nanoparticles within mesoporous titania via in situ photo-deposition. J Mater Chem 2:17321–17328
Cohen JL, Volpe DJ, Abruña HD (2007) Electrochemical determination of activation energies for methanol oxidation on polycrystalline platinum in acidic and alkaline electrolytes. Phys Chem Chem Phys 9:49–77
Muench F, Felix E-M, Rauber M, Schaefer S, Antoni M, Kunz U, Kleebe H-J, Trautmann C, Ensinger W (2016) Electrodeposition and electroless plating of hierarchical metal superstructures composed of 1d nano- and microscale building blocks. Electrochim Acta 202:47–54
Tian M, Guosheng W, Chen A (2012) Unique electrochemical catalytic behaviour of Pt nanoparticles deposited on TiO\(_{2}\) nanotubes. ACS Catal 2:425–432
Ting C-C, Liu C-H, Tai C-Y, Hsu S-C, Chao C-S, Pan F-M (2015) The size effect of titania-supported Pt nanoparticles on the electrocatalytic activity towards methanol oxidation reaction primarily via the bifunctional mechanism. J Power Sources 280:166–172
Xing L, Jia J, Wang Y, Zhang B, Dong S (2010) Pt modified TiO\(_{2}\) nanotubes electrode: preparation and electrocatalytic application for methanol oxidation. Int J Hydrogen Energy 35:12169–12173
Muench F, Bohn S, Rauber M, Seidl T, Radetinac A, Kunz U, Lauterbach S, Kleebe H-J, Trautmann C, Ensinger W (2014) Polycarbonate activation for electroless plating by dimethylaminoborane absorption and subsequent nanoparticle deposition. Appl Phys A 116:287–294
Muench F, Eils A, Toimil-Molares ME, Hossain UH, Radetinac A, Stegmann C, Kunz U, Lauterbach S, Kleebe H-J, Ensinger W (2014) Polymer activation by reducing agent absorption as a flexible tool for the creation of metal films and nanostructures by electroless plating. Surf Coat Technol 242:100–108
Felix E-M, Antoni M, Pause I, Schaefer S, Kunz U, Weidler N, Muench F, Ensinger W (2016) Template-based synthesis of metallic Pd nanotubes by electroless deposition and their use as catalysts in the 4-nitrophenol model reaction. Green Chem 18:558–564
Felix E-M, Muench F, Ensinger W (2014) Green plating of high aspect ratio gold nanotubes and their morphology-dependent performance in enzyme-free peroxide sensing. RSC Adv 4:24504–24510
Boehme M, Fu G, Ionescu E, Ensinger W (2010) Fabrication of anatase titanium dioxide nanotubes by electroless deposition using polycarbonate for separate casting method. Nano-Micro Lett 2(1):26–30
Zhang C, Hongmei Y, Li Y, Li F, Gao Y, Song W, Shao Z, Yi B (2013) Simple synthesis of Pt/TiO\(_{2}\) nanotube arrays with high activity and stability. J Electroanal Chem 701:14–19
Wiberg N (2001) Holleman–Wiberg’s inorganic chemistry. Academic Press, New York
Cornelius TW, Apel PY, Schiedt B, Trautmann C, Toimil-Molares ME, Karim S, Neumann R (2007) Investigation of nanopore evolution in ion track-etched polycarbonate membranes. Nucl Instrum Methods Phys Res B 265:553–557
Sertova N, Balanzat E, Toulemonde M, Trautmann C (2009) Investigation of initial stage of chemical etching of ion tracks in polycarbonate. Nucl Instrum Methods Phys Res B 267:1039–1044
Kundu MK, Sadhukhan M, Barman S (2015) Ordered assemblies of silver nanoparticles on carbon nitride sheets and their application in the non-enzymatic sensing of hydrogen peroxide and glucose. J Mater Chem B 3:1289–1300
Yang Z, Qi C, Zheng X, Zheng J (2015) Facile synthesis of silver nanoparticle-decorated graphene oxide nanocomposites and their application for electrochemical sensing. New J Chem 39:9358–9362
Abd-Ellah M, Moghimi N, Zhang L, Thomas JP, McGillivray D, Srivastava S, Leung KT (2016) Plasmonic gold nanoparticles for ZnO-nanotube photoanodes in dye-sensitized solar cell application. Nanoscale 8:1658–1664
Kim EY, Kumar D, Khang G, Lim D-K (2015) Recent advances in gold nanoparticle-based bioengineering applications. J Mater Chem B 3:8433–8444
Schaefer S, Felix E-M, Muench F, Antoni M, Lohaus C, Brötz J, Kunz U, Gärtner I, Ensinger W (2016) NiCo nanotubes plated on Pd seeds as a designed magnetically recollectable catalyt with high noble metal utilisation. RSC Adv 6:70033–70039
Pozio A, De Francesco M, Cemmi A, Cardellini F, Giorgi L (2002) Comparison of high surface Pt/C catalysts by cyclic voltammetry. J Power Sources 105:13–19
Mayrhofer KJJ, Strmcnik D, Blizanac BB, Stamenkovic V, Arenz M, Markovic NM (2008) Measurement of oxygen reduction activities via the rotating disc electrode method: from Pt model surfaces to carbon-supported high surface area catalyst. Electrochim Acta 53:3181–3188
Wang L, Yamauchi Y (2009) Facile synthesis of three-dimensional dendritic platinum nanoelectrocatalyst. Chem Mater 21:3562–3569
Hua H, Chenguo H, Zhao Z, Liu H, Xie X, Xi Y (2013) Pt nanoparticles supported on submicrometer-sized TiO\(_{2}\) spheres for effective methanol and ethanol oxidation. Electrochim Acta 105:130–136
Cherstiouk OV, Gavrilov AN, Plyasova LM, Molina IY, Tsirlina GA, Savinova ER (2008) Influence of structural defects on the electrocatalytic activity of platinum. J Solid State Electrochem 12:497–509
Antolini E (2007) Catalysts for direct ethanol fuel cells. J Power Sources 170:1–12
Camara GA, Iwasita T (2005) Parallel pathways of ethanol oxidation: the effect of ethanol concentration. J Electroanal Chem 578:315–321
Hitmi H, Belgsir EM, Léger J-M, Lamy C, Lezna RO (1994) A kinetic analysis of the electro-oxidation of ethanol at a platinum electrode in acid medium. Electrochim Acta 39(3):407–415
Bönnemann H, Brijoux W (1996) Catalytically active metal powders and colloids. In: Active materials, pp 339–379. VCH Verlagsgesellschaft mbH
Liu Z, Hong L, Tham MP, Lim TH, Jiang H (2006) Nanostructured Pt/C and Pd/C catalysts for direct formic acid fuel cells. J Power Sources 161:831–835
Perales-Rondón JV, Solla-Guln J, Herrero E, Sánchez-Sánchez CM (2017) Enhanced catalytic activity and stability for the electrooxidation of formic acid on lead modified shape controlled platinum nanoparticles. Appl Catal B 201:48–57
Jiang R, Li B, Fang C, Wang J (2014) Metal/semicondutor hybrid nanostructures for plasmon enhanced applications. Adv Mater 26:5274–5309
Zhao Y, Sun L, Xi M, Feng Q, Jiang C, Fong H (2014) Electrospun TiO\(_2\) nanofelt surface-decorated with Ag nanoparticles as sensitive and UV-cleanable substrate for surface enhanced Raman scattering. ACS Appl Mater Interfaces 6:5759–5767
Acknowledgements
We wish to thank the German Federal Ministry of Education and Research and the VDI/VDE IT for financial support (Project 1D-SENSE, FKZ 16ES0289K). Additionally, we would like to thank Prof. C. Trautmann and the material research group (GSI Helmholzzentrum für Schwerionenforschung GmbH) for the irradiation experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Antoni, M., Muench, F., Kunz, U. et al. Electrocatalytic applications of platinum-decorated TiO2 nanotubes prepared by a fully wet-chemical synthesis. J Mater Sci 52, 7754–7767 (2017). https://doi.org/10.1007/s10853-017-1035-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-017-1035-4