Abstract
Nonstoichiometry lead-free 0.94Na x Bi y TiO3–0.06Ba z TiO3 (N x B y T–0.06B z T) (from x = y = 0.5, z = 1.00 to x = 0.5, y = 0.534, z = 1.02) ceramic compositions were prepared by a conventional solid-state route. XRD shows that the compositions are at a morphotropic phase boundary where rhombohedral and tetragonal phases coexist. The depolarization temperature (T d) can be lowered by modifying x, y and z. The pyroelectric coefficient (p) of nonstoichiometry N x B y T–0.06B z T compositions is greatly increased, compared with stoichiometry NBT–0.06BT composition, from 3.15 × 10−4 C m−2 °C−1 at room temperature (RT) and 23.9 × 10−4 C m−2 °C−1 at T d, and reaches maxima of 6.99 × 10−4 C m−2 °C−1 at RT and 75.3 × 10−4 C m−2 °C−1 at T d for x = y = 0.52 and z = 1. The figures of merits, F i, F v, and F D, also have been improved from 1.12 × 10−10 m v−1 and 0.021 m2 C−1 to 2.50 × 10−10 m v−1, 0.047 m2 C−1 and 16.63 × 10−6 Pa−1/2, respectively, for N0.52B0.52T–0.06BT composition at RT. Furthermore, N0.52B0.52T–0.06BT composition shows a huge enhancement in F i, F v and F D to 26.9 × 10−10 m v−1, 0.39 × 10−10 m2 C−1 and 138.7 × 10−6 Pa−1/2, respectively, at T d. The same composition also presents F C values which are ~2.58 and ~2.86 (×10−9 C cm−2 °C−1) at RT at 100 and 1000 (Hz). N0.5B0.534T–0.06BT and N0.5B0.534T–0.06B1.02T compositions show a large p values at a wide temperature range. The enhanced pyroelectric properties make nonstoichiometry N0.52B0.52T–0.06BT composition a promising candidate for pyroelectric and other applications at wide temperatures range.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pyroelectric ceramic materials have been used as an active element in various electronic devices [1, 2]. These materials are playing a major role in diverse applications such as sensors, infrared detectors, thermal cameras, remote medical diagnostic devices, gas detectors, and many other applications. PZT-based multicomponent ceramic materials are the most widely utilized in electronic applications due to their superior ferroelectric, pyroelectric, and piezoelectric properties, but environmental issues relating to the hazardous nature of lead-containing materials and its disposal are driving the need to find lead-free ceramic materials as an alternative to fill the industrial gap [1,2,3].
Lead zirconate titanate (PZT) and PZT-based materials exhibit exceptional ferroelectric properties around the morphotropic phase boundary (MPB). Consequently, effort has focused on alternative lead-free materials that possess an MPB region as replacements for PZT-based materials, and such materials have been explored in the past few decades [2]. (1 − x)(Na0.5Bi0.5)TiO3–xBaTiO3 (NBT–xBT) as a lead-free system has attracted interest of numerous researchers due to its high ferroelectric properties at the MPB at x = 0.06–0.07 [1, 2, 4, 5], and the lower coercive field here which makes poling easier [5]. The phase diagram of NBT–0.06BT is complex and presents many phases. Firstly, from ferroelectric (FE) to antiferroelectric (AFE) or to relaxor at depolarization temperature (T d), it is reported at around 100 to ~165 °C [6,7,8,9,10,11,12,13]. Secondly, from antiferroelectric (AFE) to nonpolar (PE) phases, it happens at around 225–292 °C, at either Curie temperature (T c) or the temperature at the maximum dielectric permittivity (T m) [6,7,8,9,10, 12]. The NBT–0.06BT phase transitions are also associated with structural changes, which can be either from rhombohedral (Rh) and tetragonal (Tr), if the composition is at the MPB, or from rhombohedral (Rh), if the composition is out of MPB, to tetragonal (Tr), and from tetragonal (Tr) to cubic (C) [9, 11, 12]. These structural phase changes are very important to the pyroelectric applications because large pyroelectric effects can be induced at these transitions.
NBT–0.06BT has been extensively investigated for dielectric, ferroelectric, and piezoelectric properties but to a lesser extent in terms of its pyroelectric properties [7, 9, 14, 15]. In previous work, we investigated the effects of Ba2+ content on the pyroelectric properties of NBT–0.06B(1+x)T ceramics [2] and found that p increased from 2.90 to 3.54 (×10−4 C m−2 C−1) at RT when x = 1.02. In addition, p showed huge enhancement from 55.3 to 740.7 (×10−4 C m−2 C−1) at T d (85 °C) for the composition NBT–0.06B1.02TiO3 [16]. Follow on studies on this work, reported the effects of Ta doping in NBT–0.06BT with the pyroelectric coefficient highly enhanced from 3.14 to 7.14 (×10−4 C m−2 C−1) at RT at Ta = 0.2% with the same composition showing a maximum value of 146.10 × 10−4 C m−2 C−1 at T d (78.8 °C) [16]. Furthermore, our recent investigation of NBT–0.06BT doped with La reveals that the pyroelectric properties of NBT–0.06BT were greatly improved by La doping from 3.14 to 7.42 (×10−4 C m−2 °C−1) at RT at La = 0.5% and to 105.40 × 10−4 C m−2 °C−1 at T d (67.9 °C) at La = 0.2% [17].
Pyroelectric properties usually reach a peak value at phase transition temperatures, such as T d and T c. These two phase transition temperatures in NBT–0.06BT are far too high for practical use at room temperature, so it would be beneficial if these phase transition temperatures can be lowered. In this study, we expect to bring down the depolarization temperature by modifying the stoichiometric state of the compositional contents x, y, and z of NBT–0.06BT, to simultaneously improve the pyroelectric properties of these compositions at both RT and T d.
Experimental
A solid-state synthesis technique was selected to prepare the stoichiometry 0.94NBT–0.06BT (NBT–0.06BT) and nonstoichiometry 0.94Na x Bi y TiO3–0.06Ba z TiO3 (Na x Bi y T–0.06Ba z T) compositions. The reagents used in this project were bismuth oxide (Bi2O3), 99.999%, sodium carbonate (Na2CO3), 99.5%, barium carbonate (BaCO3), 99.98%, and titanium dioxide (TiO2), >99.8% (all Sigma-Aldrich).
The amounts of powders were calculated according to the chemical formula of 0.94Na x Bi y TiO3–0.06Ba z TiO3, with the x, y, and z values for each composition listed in Table 1, where the start composition of B is modified by receive an extra 0.02 from Na(x) and Bi(y) concentrations and the composition of C is also changed by modify the y/x ratio to be >1, while the start composition of D is as same as composition C with extra Ba (z) 0.02 concentration, as given in Table 1. The raw materials were ball-milled (zirconia milling media) in acetone in polyethylene pots for 24 h in order to mix and mill the powders. The resultant slurries were dried overnight at 50 °C, and the dried powder cakes were ground in a mortar for 10 min and sieved through a 250-μm mesh in order to aid the calcination step. The powders were calcined at 850 °C for 180 min in a closed alumina crucible with a heating ramp rate of 1 °C per minute and cooling rate 5 °C per minute in a furnace (Pyro Therm, ITEMP 14/6). After calcination, the powders were re-milled in acetone for 24 h, and then, 2% (wt.) of poly vinyl alcohol (PVA) was added as an organic binder to the dried powders to enhance the mechanical strength of the pellets during pressing. After that, the powders were fully dried in oven at 80 °C. The dried powders were ground and sieved and subsequently pressed into green pellets with a diameter of 10 mm under an uniaxial compaction with a load of ~78MPs for 5 min at RT. The pellets were sintered at temperatures up to 1150 °C for 120 min in closed crucibles in order to minimize the loss of volatile Na+ and Bi3+. The pellets were lapped/polished on both sides using silicon carbide paper. Silver conductive paint (RS limited) was used to electrode the pellets, and electrical poling at 6.5 kV/mm for 10 min at RT in mineral oil was carried out using a Keithley (6517 electrometer/high resistance) DC power supply. Afterwards, the poled samples were washed thoroughly by using isopropanol in order to remove the mineral oil and then left in air for 3 h to dry. The poled samples were placed at RT for 60 min with their electrodes short-circuited to eliminate any unwanted trapped charges.
SEM (FEI XL30 SFEG) was used to look at the grain morphology of the sintered samples, and X-ray diffraction (XRD) [Siemens Ltd Model: D500) was used to investigate crystallization and phase. Dielectric measurements were performed on an impedance analyser (Wayne kerr Electronics Ltd. Model 3245 and Hewlett Packard HP4092A)] over a temperature range from RT to 150 °C using a custom-built temperature-controlled hotplate in the frequency range of 0.1–10 kHz. Pyroelectric measurements were made using the Byer–Roundy [18] method on a custom-built computer-controlled rig which used thermoelectric heaters to ramp the temperature between 20 and 90 °C while under vacuum and collect the pyroelectric current response from a Keithley electrometer (Model 6217). Dielectric and pyroelectric data were then used to study the phase changes such as depolarization temperatures (T d) and to determine figure of merit values F i, F v, F D, and F C.
Results and discussion
Effects of nonstoichiometry composition on microstructure
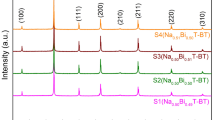
Figure 1a depicts the XRD spectra of stoichiometry and nonstoichiometry ceramic powders calcined at 850 °C for 3 h. All the compositions present a single-crystalline perovskite structure with no evidence of any secondary phases indicating that the excess Na+, Bi3+, and Ba2+ diffused into the NBT–0.06BT main lattice and formed the new nonstoichiometry N x B y T–0.06B z T ceramic solid solutions [19, 20].
Figure 1b illustrates a splitting of the peak [111] into [003] and [021] at 39.0°–41.0°, for all the compositions showing the existence of the rhombohedral (Rh) phase. This split coincides with that observed in the X-ray diffraction spectra of the unmodified or undoped NBT [11, 19].
A similar splitting was identified in all compositions for the [200] peak into [200] and [002] at 46.0°–48.0° as shown in Fig. 1c, which indicates the presence of tetragonal (Tr) phase in the N x B y T–0.06B z T lattice [11, 20, 21]. The coexistence of both Rh and Tr phases in the N x B y T–0.06B z T ceramics confirms that the structures of stoichiometry composition (A) are at the MPB and also the nonstoichiometry compositions (B, C, and D) remain at MPB area. Although the stoichiometric state of B, C, and D has been changed by modifying the elements molar concentrations (x, y, and z) in this study as mention before, XRD patterns show no significant changes in the phase structure of nonstoichiometry N x B y T–0.06B z T compositions compared with the stoichiometry composition which is in agreement with the literature [11, 19, 21, 22].
According to the ionic matching relationship [23], the ionic radius of all elements in the compositions is Na+ (1.39 Å, CN 12) (CN = coordination number), Bi3+ (1.31 Å, CN 12), Ba2+ (1.61 Å, CN12), and for Ti4+ (0.61 Å, CN 6). The excess in the elements content will compensate the loss of elements due to evaporation at the calcining and sintering stages and may also substitute other cations in the composition [24]. For instance, the excess in the barium content in Composition D may compensate the deficiency in the Ba2+, which will improve the tetragonality in the N0.5B0.534TiO3–0.06B1.02T composition [2], simultaneously being more likely to substitute the sodium than replace the bismuth in NBT due to the larger radial difference (>15% for Bi) [19].
To determine the stability of the perovskite structure, Goldschmidt tolerance factor (t) relation was calculated by using Eq. 1 [25,26,27,28]:
where R A, R B, and R o are the ionic radius of ions which will occupy A, B sites, and the oxygen anion. The tolerance factor value of stable perovskite structure is in the range of 0.77–0.99 [27]. The average t value which has been calculated for the stoichiometry and nonstoichiometry compositions in this study is around 0.982, suggesting that all compositions illustrate stable structure.
Table 1 illustrates the lattice parameters (Å), the c/a ratio, the average grains size (µm), and samples density (g cm−3) of all compositions. The lattice parameters are “a = b” and “c” vary by modifying x, y, and z contents. The c parameter shows a slight increase from composition A to D. Compositions B, C, and D have higher c/a ratio than Composition A indicating that the nonstoichiometry Compositions B, C, and D have more tetragonality in their structure. This result is in agreement with the Yoon et al. [29] results.
Figure 2 shows the SEM surface morphology of the Compositions A, B, C, and D. All the compositions display a dense microstructure with an average density of around 5.689 ± 0.264 g cm−3. Although B, C, and D have nonstoichiometry compositions with excess sodium, bismuth, and barium contents, they present a lower density value than Composition A (Table 1). Stoichiometry and nonstoichiometry compositions show a granule grain shape with various grain sizes. The average grain size reduces gradually from Compositions A to D (Table 1). The reason behind the reduction in the average grain size may link to the compositional changes that happened during calcining and sintering at high temperature. The loss of Na+ is more likely higher than that of Bi3+ due to the larger volatility of Na+. Thus, the composition may shift to a bismuth-nonstoichiometry state after calcining and sintering. Generally, the bismuth behaves as an inhibitor of the grain growth in the NBT structure. Hence, the excess in bismuth content in Composition B can reduce the grain size [11].
Both Compositions C and D are bismuth nonstoichiometric. Therefore, the effect of the Bi3+ on grain size is more pronounced in these compositions. Composition D presents the smallest grain size, which is due to the influence of Bi3+ and Ba2+ nonstoichiometric (excess content), and both of which may boost the prevention of the grain growth [2, 19, 29, 30]. In PZT ceramics, similar results (grain size reduction) have been reported where lead was substituted with barium [2, 19, 29, 30].
Effects of nonstoichiometry composition on dielectric properties
Modifying the stoichiometric state of the starting composition has a big effect on the magnitude of the dielectric properties of the N x B y T–0.06B z T material. Figure 3a–d presents the change of the dielectric properties of poled compositions from A to D as a function of temperature from 20 to 150 °C at 1, 10, and 100 (kHz). The relative permittivity (ɛ r) of all the compositions increases with temperature, which behaves like a typical ferroelectric material [31].
Relative permittivity (ɛ r) and loss tangent (tanδ) versus temperature for a NBT–0.06BT, b N0.52B0.52T-0.06BT, c N0.5B0.534T–0.06BT, and d N0.5B0.534T–0.06B1.02T at three different frequencies 1, 10, and 100 (kHz), where green dotted line ɛ r-1, black dotted line ɛ r-10, red dotted line ɛ r-100, green solid line tanδ-1, black solid line tanδ-10, red solid line tanδ-100
The relative permittivity (ɛ r) shows a significant increase with the increase in the x, y, and z contents, with values of 396, 597, 893, and 933, respectively, for the Compositions A to D at RT and 1 kHz; therefore, the small variation in start compositions can be responsible for the significant increase in the relative permittivity value [24].
The dielectric loss (tanδ) also increases with the temperature up to a certain point and then decreases. The tanδ value of Composition A is ~0.0436 at RT and 1 kHz (Fig. 3a). It increases for nonstoichiometry Compositions C and D to around 0.0531 and 0.0561, respectively (Fig. 3b–d). However, Composition B presents unexpected dielectric loss value (~0.0427) which is the lowest value among all other compositions (Fig. 3b).
The temperature at which tanδ shows a maximum value is widely known as the depolarization temperature (T d) [13, 32, 33]. The tanδ values at T d are around 0.0510, 0.0548, 0.0599, and 0.0625, and their corresponding temperatures estimated at ~115, 85, 55, and 65 (°C) for Compositions A, B, C, and D, respectively (Fig. 4). The tanδ results at T d reveal that B shows the lowest value among all other compositions. The ɛ r values at T d are ~582, 792, 1055, and 1105 for Compositions A, B, C, and D, respectively (Fig. 3a–d). The ɛ r values at T d clearly present that the change in the nonstoichiometric state of the composition has a significant effect on the dielectric properties, but the dramatic effect is on the relative permittivity at wide temperature range.
The identification of the T d values of all compositions by dielectric method [loss tangent (tanδ) against temperature (°C)] for NBT–0.06BT and N x B y T–0.06B z T at 1 kHz, where green solid line Composition A, black solid line Composition B, red solid line Composition C, blue solid line Composition D
Effects of nonstoichiometry composition on depolarization temperature
Changing the stoichiometric state of the composition can also make a large effect on the depolarization temperature (T d) of the N x B y T–0.06B z T compositions, where the Na0.49Bi0.50TiO3 shows lower T d than Na0.50Bi0.50TiO3 [24]. Although depolarization temperature is an important parameter in pyroelectric study, its origin has not been fully understood for NBT–xBT. It has more than one definition [27, 32, 34,35,36,37], which includes the phase transition temperature from ferroelectric (FE) to antiferroelectric (AFE) phases or from ferroelectric to relaxor [33, 34, 38, 39].
In this study, the T d is subjectively identified by two methods: (1) dielectric method, from the dielectric loss peak value versus temperature plots (Fig. 4), and (2) pyroelectric method, the pyroelectric coefficient (p) peak value versus temperature plots (Fig. 5) [14]. For both methods, the T d is strongly dependent on modifying x, y, and z concentrations of the compositions. Figure 4 reveals that T d decreased from 115 °C in Composition A to around 55 °C for Composition C, respectively. Composition D illustrates a higher T d, which is more than 10 °C higher than the T d for Composition C (Fig. 4), whereas a shift to lower temperature for Ba2+-nonstoichiometric composition was expected according to our previous study [2].
T d can also be obtained from the maximum pyroelectric coefficient values. It is widely known that the maximum p can be achieved at the phase transition temperature [40,41,42,43]. In this study, T d values identified by this method showed a decrease from more than 90 °C to ~82.7, 60.0, and 60.6 °C for Compositions A to D, respectively. The exact T d could not be identified for Composition A by the pyroelectric method due to the upper temperature limit of equipment (Fig. 5). In addition, Composition D shows a similar T d to Composition C although as mentioned above, a lower value with increasing the Ba2+ content [2] was expected. The Bi3+ nonstoichiometric (excess content) in Composition D may be the reason for unexpected high T d in both methods.
The decrease in T d for nonstoichiometry compositions is probably caused by the excess amount of bismuth and/or deficiency of sodium and also by extra barium concentration in the case of Composition D. According to Yoon et al. [29], Guo et al. [33], and Li et al. [44], the T d coincides with phase transition from ferroelectric to a so-called intermediate phase phase, and this transition shows a shift of T d towards lower temperature for compositions with excess Bi3+ and/or deficit of Na+. Excess Bi3+ concentration in the Compositions B, C, and D is more likely to replace the A-site ions, Na+, Bi3+, and Ba2+ rather than B-site Ti4+ [29, 44] as discussed before, which may result in the generation of some of the A-site vacancies when Bi3+ replaces Na+. These vacancies can reduce ferroelectric long-range ordering and weaken the coupling between A-site ions and TiO6 octahedron, as a result inducing the phase change at lower temperature [29, 44]. Our results agree with the literature but also reveal that the T d can be reduced and shifted towards lower temperature not only by changing the Bi3+ /Na+ ratio but also by modifying all the A-site ions (Na+, Bi3+, and Ba2+) contents [2, 24, 33].
Comparing T d values obtained by the two methods, the pyroelectric method shows T d values slightly lower than the dielectric method, which is in agreement with the literature [32].
Effects of nonstoichiometry composition on pyroelectric properties
Modifying the stoichiometry state of the start composition can significantly affect the pyroelectric coefficient (p) of NBT–0.06BT material. However, the efficient way for evaluating the pyroelectric material performance is to calculate the figure of merits (FOMs) that represent the essential parameters for the pyroelectric applications [32]. The calculation of the FOMs is based on the values of the pyroelectric coefficient, relative dielectric permittivity, dielectric loss, and the specific heat [29]. There are several types of FOMs depending on special types of the pyroelectric applications such as infrared or thermal imaging [21, 45]. The types of FOMs depend on the input of thermal or electrical circuits, current, or voltage [8, 14, 32].
In this study, the pyroelectric coefficient (p) of NBT–0.06BT and N x B y T–0.06B z T was measured from 20 to 90 °C for stoichiometric Composition (A) or T d for nonstoichiometric Compositions B, C, and D. The calculations of the pyroelectric coefficient (p) and FOMs were carried out using Eqs. 2–6 [9, 15, 18].
where T is absolute temperature, t is the time, p is the pyroelectric coefficient, I p is the pyroelectric current, F i is the high current (i) detectivity, F v is the high voltage (\( {\text{v}} \)) detectivity, F D is high the detectivity and F C is the pyroelectric figure of merit. \( C_{v} \) * is the specific heat (2.8 J K−1 cm−3, quoted from Ref. [14]), ɛ r is the relative dielectric permittivity, and ɛ o is the permittivity of free space.
Figure 5 shows the change of p versus T for all compositions from 20 to 90 °C, with Composition B showing the highest p value at RT. In the temperature range from 33 to 70 °C, Compositions C and D have similar pyroelectric coefficient values with a very close maximum value of p. In contrast, Composition B shows a very characteristic peak p value. Generally, pyroelectric materials show one sharp peak at a phase transition temperature as with Composition B. However, it is very evident that the broad flat profiles for Compositions C and D in the temperature range (33–70 °C) must be linked to a different compositional structure. The composition of both samples is located at the MPB. At T d, their structure has been changed to tetragonal with antiferroelectric or relaxor state. However, with the increase in temperature, the structure and polarization states possibly changed from rhombohedral and tetragonal coexisting (MPB) ferroelectric to tetragonal ferroelectric and then to tetragonal antiferroelectric or relaxor. This phase transition may give the pyroelectric coefficient with a broad flat peak temperature region.
The p, and FOMs F i, F v, and F D values for all compositions are listed in Table 2 at RT, while those values at 90 °C or T d are listed in Table 3.
The pyroelectric coefficients of nonstoichiometric compositions vary but are higher than Composition A at RT and T d.
Composition B shows the optimum p value at both RT and T d (Tables 2 and 3). This may be linked to the microstructural change due to the change of the stoichiometric state of this composition. Composition B has a bigger c/a ratio than all other compositions (Table 1), indicating that Composition B has more tetragonality in its structure than all other compositions [13, 43, 44, 46, 47]. Importantly, at RT, the p values for nonstoichiometry compositions are higher than that of PZT and other lead-free compositions (Table 2) [7, 19, 27, 32].
The F i, F v, and F D show also an increasing trend as p at RT. Compared with PZT and other lead-free compositions (Table 2) [7, 9, 29, 30, 42], the nonstoichiometry compositions have higher F i, F v, and F D values at RT with Composition B showing the highest value at RT and T d (Tables 2 and 3) from this study. The optimum F v and F D values (Tables 2 and 3) were observed for Composition B at both RT and T d, which can be linked to the lower ɛ r and the higher p values observed at these temperatures compared to Compositions C and D. Also the stoichiometry composition A has a better F D value than C and D due mainly to the increases in ɛ r and tanδ in this nonstoichiometry compositions.
The F C values of stoichiometry and nonstoichiometry compositions at RT and at two frequencies 100 and 1000 (Hz) were calculated (Table 4). The F C values revealed a strong dependence upon x, y, and z contents. Generally, the materials suitable for infrared detectors should have a F C threshold value ~3 ± 1 × 10−9 C cm−2 °C−1 [15]. Composition B shows F C values which are within the threshold range. Compositions A, C, and D present lower and close to that the RodrÍguez-Ruiz et al. [15] founded for a 0.935Na0.5Bi0.5TiO3–0.065BaTiO3 composition which is around 1.44 × 10−9 C cm−2 °C−1. Thus, the composition of Composition B is a promising candidate for this particular pyroelectric application.
Conclusion
The stoichiometry NBT–0.06BT and nonstoichiometry N x B y T–0.06B z T ceramic compositions were prepared by a conventional solid-state technique. The pyroelectric properties of these compositions were investigated, and their values were better than those in PZT and other lead-free ceramic compositions.
XRD indicates that all compositions phase structures are at the MPB region. The lattice parameter values and c/a ratio slightly change in nonstoichiometry compositions. The average grain size reduces from 1.79 (A) to 1.41 µm (D). Moreover, the stoichiometry composition shows a higher density than the nonstoichiometry compositions. Both relative permittivity (ɛ r) and dielectric loss (tanδ) are affected by change in the stoichiometric state of the compositions and show an increase with the increase in x, y, and z contents.
The T d was subjectively identified by two different methods and decreases from ~115 °C in Composition A to around 55 °C in Composition C, as predicted by the dielectric method. However, the T d value for Composition C was ~60 °C according to pyroelectric coefficient method which questions the accuracy of the two techniques to give a definitive answer. However, it was evident that certain compositions showed much clearer maxima to pinpoint the T d, compared to others, which suggests that the techniques may still be valid.
Composition B has the optimum p at RT and T d. The F i, F v , and F D increase with the increase in Na+ (x) and Bi3+ (y) contents up to x = y = 0.52 at both RT and T d. The F C shows the optimum values in Composition B. The results presented in this study demonstrate that the nonstoichiometry N0.52B0.52T–0.06BT composition is a promising material for infrared detectors and other pyroelectric applications in a wide range of temperatures and frequencies.
References
García JE (2016) Elastic, dielectric and electromechanical properties of (Bi0.5Na0.5)TiO3–BaTiO3 piezoceramics at the morphotropic phase boundary region. J Alloys Compd. doi:10.1016/j.jallcom.2016.08.116
Balakt AM, Shaw CP, Zhang Q (2016) The effects of Ba2+ content on depolarization temperature and pyroelectric properties of lead-free 0.94Na0.5Bi0.5TiO3–0.06Ba1 + xTiO3 ceramics. J Mater Sci Mater Electron 27:12947–12954. doi:10.1007/s10854-016-5433-1
Jo W, Schaab S, Sapper E et al (2011) On the phase identity and its thermal evolution of lead free (Bi1/2Na1/2)TiO3–6 mol BaTiO3. J Appl Phys. doi:10.1063/1.3645054
Ma C, Tan X (2010) Phase diagram of unpoled lead-free-ceramics. Solid State Commun 150:1497–1500. doi:10.1016/j.ssc.2010.06.006
McQuade RR, Dolgos MR (2015) A review of the structure-property relationships in lead-free piezoelectric (1 − x)Na0.5Bi0.5TiO3–(x)BaTiO3. J Solid State Chem 3:1–8. doi:10.1016/j.jssc.2016.01.008
Cheng SY, Shieh J, Lu HY et al (2013) Structure analysis of bismuth sodium titanate-based A-site relaxor ferroelectrics by electron diffraction. J Eur Ceram Soc 33:2141–2153. doi:10.1016/j.jeurceramsoc.2013.03.020
Guo F, Yang B, Zhang S et al (2013) Enhanced pyroelectric property in (1 − x)(Bi0.5Na0.5)TiO3–xBa(Zr0.055Ti0.945)O3: role of morphotropic phase boundary and ferroelectric-antiferroelectric phase transition. Appl Phys Lett 103:182906. doi:10.1063/1.4828675
Lau ST, Cheng CH, Choy SH et al (2008) Lead-free ceramics for pyroelectric applications. J Appl Phys 103:10–13. doi:10.1063/1.2927252
Martínez FL, Hinojosa J, Doménech G et al (2013) Dielectric constant tunability at microwave frequencies and pyroelectric behavior. IEEE Trans Ultrason Ferroelectr Freq Control 60:1595–1602. doi:10.1109/TUFFC.2013.2740
Razak KA, Yip CJ, Sreekantan S (2011) Synthesis of (Bi0.5Na0.5)TiO3 (BNT) and Pr doped BNT using the soft combustion technique and its properties. J Alloys Compd 509:2936–2941. doi:10.1016/j.jallcom.2010.11.163
Chen M, Xu Q, Kim BH et al (2008) Structure and electrical properties of (Na0.5Bi0.5)1 − xBaxTiO3 piezoelectric ceramics. J Eur Ceram Soc 28:843–849. doi:10.1016/j.jeurceramsoc.2007.08.007
Kanuru SR, Baskar K, Dhanasekaran R (2016) Synthesis, structural, morphological and electrical properties of NBT–BT ceramics for piezoelectric applications. Ceram Int 42:6054–6064. doi:10.1016/j.ceramint.2015.12.162
Jo W, Daniels J, Damjanovic D et al (2013) Two-stage processes of electrically induced-ferroelectric to relaxor transition in 0.94(Bi1/2Na1/2)TiO3–0.06BaTiO3. Appl Phys Lett 102:192903. doi:10.1063/1.4805360
Abo J, Kobune M, Nishimura T, Yazaw T, Nakai Y (2006) Effects of manganese addition on pyroelectric properties of (Bi0.5Na0.5TiO3) 0.94 (BaTiO3) 0.06 ceramics. Integr Ferroelectr 80:87–95. doi:10.1080/10584580600656502
RodrÍguez-Ruiz R, González-Ballesteros R, Flores-Cuautle A, Suaste-Gómez E (2008) Determination of the pyroelectric coefficient for (Bi0.5Na0.5)0.935 Ba0.065 TiO3 piezoelectric ceramics. Ferroelectrics 368:216–223. doi:10.1080/00150190802368537
Balakt AM, Shaw CP, Zhang Q (2016) The decrease of depolarization temperature and the improvement of pyroelectric properties by doping Ta in lead-free 0.94Na0.5Bi0.5TiO3–0.06BaTiO3 ceramics. Ceram Int. doi:10.1016/j.ceramint.2016.12.004
Balakt AM, Shaw CP, Zhang Q (2016) Enhancement of pyroelectric properties of lead-free 0.94Na0.5Bi0.5TiO3–0.06BaTiO3 ceramics by La doping. J Eur Ceram Soc. doi:10.1016/j.jeurceramsoc.2016.12.021
Byer RL, Roundy CB (1972) Pyroelectric coefficient direct measurement technique and application to a nsec response time detector. IEEE Trans Sonics Ultrason 19:333–338. doi:10.1109/T-SU.1972.29679
Xu C, Lin D, Kwok KW (2008) Structure, electrical properties and depolarization temperature of (Bi0.5Na0.5)TiO3–BaTiO3 lead-free piezoelectric ceramics. Solid State Sci 10:934–940. doi:10.1016/j.solidstatesciences.2007.11.003
Rawat M, Yadav KL, Kumar A et al (2012) Structural, dielectric and conductivity properties of Ba2+ doped (Bi0.0.5)TiO3 ceramic. Adv Mater Lett 3:286–292. doi:10.5185/amlett.2012.2322
Li Q, Wang J, Ma L et al (2016) Large electrocaloric effect in (Bi0.5Na0.5)0.94Ba0.06TiO3 lead-free ferroelectric ceramics by La2O3 addition. Mater Res Bull 74:57–61. doi:10.1016/j.materresbull.2015.10.010
Search H, Journals C, Contact A, Iopscience M (1991) (Bi1/2Na1/2) TiO3–BaTiO3 system for lead-free piezoelectric ceramics. Jpn J Appl Phys 2236:2236–2239
Shannon RD (1976) Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr Sect A 32:751–767. doi:10.1107/S0567739476001551
Li M, Zhang H, Cook SN et al (2015) Dramatic influence of A-site nonstoichiometry on the electrical conductivity and conduction mechanisms in the perovskite oxide Na0.5Bi0.5TiO3. Chem Mater 27:629–634. doi:10.1021/cm504475k
Hiruma Y, Nagata H, Takenaka T (2009) Formation of morphotropic phase boundary and electrical properties of (Bi1/2Na1/2)TiO3–Ba(Al1/2Nb1/2)O3 solid solution ceramics. Jpn J Appl Phys 48:09KC08. doi:10.1143/JJAP.48.09KC08
Kumari R, Ahlawat N, Agarwal A et al (2016) Structural transformation and investigation of dielectric properties of Ca substituted (Na0.5Bi0.5)0.95 − xBa0.05CaxTiO3 ceramics. J Alloys Compd. doi:10.1016/j.jallcom.2016.11.200
Cho J-H, Jeong Y-H, Nam J-H et al (2014) Phase transition and piezoelectric properties of lead-free (Bi1/2Na1/2)TiO3–BaTiO3 ceramics. Ceram Int 40:8419–8425. doi:10.1016/j.ceramint.2014.01.051
Le Goupil F, Bennett J, Axelsson AK et al (2015) Electrocaloric enhancement near the morphotropic phase boundary in lead-free NBT–KBT ceramics. Appl Phys Lett 107:103–107. doi:10.1063/1.4934759
Yoon MS, Khansur NH, Ur SC (2010) The effect of pre-milling/pre-synthesis process and excess Ba on the microstructure and dielectric/piezoelectric properties of nano-sized 0.94[(Bi0.5Na0.5)TiO3]–0.06[Ba(1 + x)TiO3]. Ceram Int 36:1265–1275. doi:10.1016/j.ceramint.2010.01.011
Hiruma Y, Yoshii K, Nagata H, Takenaka T (2007) Investigation of phase transition temperatures on (Bi1/2Na1/2)TiO3–(Bi1/2K1/2)TiO3 and (Bi1/2Na1/2)TiO3–BaTiO3 lead-free piezoelectric ceramics by electrical measurements. Ferroelectrics 346:114–119. doi:10.1080/00150190601180471
Guggilla P, Batra AK, Currie JR et al (2006) Pyroelectric ceramics for infrared detection applications. Mater Lett 60:1937–1942. doi:10.1016/j.matlet.2005.05.086
Anton EM, Jo W, Damjanovic D, Rdel J (2011) Determination of depolarization temperature of (Bi1/2Na 1/2)TiO3-based lead-free piezoceramics. J Appl Phys 110:1–14. doi:10.1063/1.3660253
Guo Y, Gu M, Luo H (2011) Antiferroelectric phase and pyroelectric response in (NayBiz)Ti1 − xO3(1 − x)–xBaTi3 ceramics. J Am Ceram Soc 94:1350–1353. doi:10.1111/j.1551-2916.2011.04509.x
Sapper E, Schaab S, Jo W et al (2012) Influence of electric fields on the depolarization temperature of Mn-doped (1 − x)Bi1/2Na1/2TiO3–xBaTiO3. J Appl Phys 111:14105. doi:10.1063/1.3674275
Levanyuk AP (2015) Phase transitions and domain structures in thin ferroelectric films. Phase Transit 88:682–691. doi:10.1080/01411594.2014.986732
Lichtensteiger C, Fernandez-Pena S, Weymann C et al (2014) Tuning of the depolarization field and nanodomain structure in ferroelectric thin films. Nano Lett 14:4205–4211. doi:10.1021/nl404734z
Okatan MB, Mantese JV, Alpay SP (2010) Effect of space charge on the polarization hysteresis characteristics of monolithic and compositionally graded ferroelectrics. Acta Mater 58:39–48. doi:10.1016/j.actamat.2009.08.055
Ranjan R, Dviwedi A (2005) Structure and dielectric properties of (Na0.50Bi0.50)1 − xBaxTiO3: 0 ≤ x ≤ 0.10. Solid State Commun 135:394–399. doi:10.1016/j.ssc.2005.03.053
Zhang S, Kounga AB, Aulbach E et al (2008) Lead-free piezoceramics with giant strain in the system Bi0.5Na0.5TiO3–BaTiO3–K0.5Na0.5NbO3. II. Temperature dependent properties. J Appl Phys 103:34108. doi:10.1063/1.2838476
Anem S, Rao KS, Rao KH (2016) Investigation of lanthanum substitution in lead-free BNBT ceramics for transducer applications. Ceram Int. doi:10.1016/j.ceramint.2016.06.173
Kang W-S, Lee S-K, Koh J-H (2015) AC conductivity and dielectric properties of (Bi, Na)TiO3–BaTiO3 lead free ceramics. Ceram Int 41:6925–6932. doi:10.1016/j.ceramint.2015.01.147
Wang X, Tang X, Chan H (2004) Electromechanical and ferroelectric properties of (Bi1/2Na1/2) TiO3–(Bi1/2K1/2) TiO3–BaTiO3 lead-free piezoelectric ceramics. Appl Phys Lett 85:91. doi:10.1063/1.1767592
Yao Z, Chu R, Xu Z et al (2016) Processing and enhanced electrical properties of Sr1 − x(K0.5Bi0.5)xBi2Nb2O9 lead-free piezoelectric ceramics. Ceram Int 42:10619–10623. doi:10.1016/j.ceramint.2016.03.156
Li XJ, Xi ZZ, Long W, Fang PY (2013) Synthesis of antiferroelectric (Bi0.534Na0.5)0.94Ba0.06TiO3 ceramics with high phase transition temperature and broad temperature range by a solid-state reaction method. Chin Sci Bull 58:2893–2897. doi:10.1007/s11434-013-5972-2
Xu Q, Huang DP, Chen M et al (2009) Effect of bismuth excess on ferroelectric and piezoelectric properties of a (Na0.5Bi0.5)TiO3–BaTiO3 composition near the morphotropic phase boundary. J Alloys Compd 471:310–316. doi:10.1016/j.jallcom.2008.03.078
Craciun F, Galassi C, Birjega R (2012) Electric-field-induced and spontaneous relaxor-ferroelectric phase transitions in (Na1/2Bi1/2)1 − XBa xTiO3. J Appl Phys. doi:10.1063/1.4770326
Patel S, Chauhan A, Kundu S et al (2015) Tuning of dielectric, pyroelectric and ferroelectric properties of 0.715Bi0.5Na0.5TiO3–0.065BaTiO3–0.22SrTiO3 ceramic by internal clamping. AIP Adv 5:87145. doi:10.1063/1.4929328
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare that we have no conflict of interest because there is no funding received for this work.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Balakt, A.M., Shaw, C.P. & Zhang, Q. Large pyroelectric properties at reduced depolarization temperature in A-site nonstoichiometry composition of lead-free 0.94Na x Bi y TiO3–0.06Ba z TiO3 ceramics. J Mater Sci 52, 7382–7393 (2017). https://doi.org/10.1007/s10853-017-0973-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-017-0973-1