Abstract
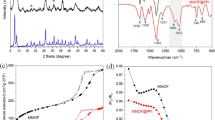
The optimal storage period/condition and the number of repeated use with regenerations were studied in this research in order to fully utilize magnetic-cored dendrimers (MDs) terminalized with amine groups as adsorbents. MDs terminalized with amine groups were synthesized and characterized with X ray diffraction, scanning electron microscopy, Fourier transform infrared spectroscopy, thermo-gravimetric analysis, surface area analysis, vibrating sample magnetometry, and zeta potential analysis to confirm successful synthesis. The order of adsorption affinity to the MDs was Pb2+ > Cd2+. The effective period of the synthesized MDs was 56 days, and the storage condition for the MDs was in a dry vial. Hydrochloric acid was chosen as the desorption agent to evaluate the regeneration efficiency. After seven regeneration cycles, the adsorption efficiency was approximately 80 %, but it gradually decreased to 55 % after fifteen cycles due to insufficient desorption of loaded heavy metal and loss of adsorbent in the acidic condition during the desorption process. Iron leaching from the MD cores and deterioration of organic branches during desorption were the main reasons for the adsorbent loss. The fraction of iron leaching was dependent on the acidity while the organic carbon leaching fraction was not severely affected by the different hydrochloric acid concentrations.












Similar content being viewed by others
References
Satheeshkumar C, Ravivarma M, Rajakumar P, Ashokkumar R, Jeong D-C, Song C (2015) Synthesis, photophysical and electrochemical properties of stilbenoid dendrimers with phenothiazine surface group. Tetrahedron Lett 56(2):321–326
Duran-Lara EF, Marple JL, Giesen JA, Fang Y, Jordan JH, Godbey WT et al (2015) Investigation of lysine-functionalized dendrimers as dichlorvos detoxification agents. Biomacromolecules 16(11):3434–3444
Jiang Y, Gao Q, Yu H, Chen Y, Deng F (2007) Intensively competitive adsorption for heavy metal ions by PAMAM-SBA-15 and EDTA-PAMAM-SBA-15 inorganic–organic hybrid materials. Microporous Mesoporous Mater 103(1–3):316–324
Sun C, Qu R, Ji C, Wang C, Sun Y, Yue Z et al (2006) Preparation and adsorption properties of crosslinked polystyrene-supported low-generation diethanolamine-typed dendrimer for metal ions. Talanta 70(1):14–19
Wang Q, Guan Y, Liu X, Ren X, Yang M (2012) High-capacity adsorption of hexavalent chromium from aqueous solution using magnetic microspheres by surface dendrimer graft modification. J Colloid Interface Sci 375(1):160–166
Niu Y, Qu R, Sun C, Wang C, Chen H, Ji C et al (2013) Adsorption of Pb(II) from aqueous solution by silica-gel supported hyperbranched polyamidoamine dendrimers. J Hazard Mater 244–245:276–286
Golikand AN, Didehban K, Irannejad L (2012) Synthesis and characterization of triazine-based dendrimers and their application in metal ion adsorption. J Appl Polym Sci 123(2):1245–1251
Anbia M, Haqshenas M (2015) Adsorption studies of Pb(II) and Cu (II) ions on mesoporous carbon nitride functionalized with melamine-based dendrimer amine. Int J Environ Sci Technol 12(8):2649–2664
Zhao J, Zhang X, He X, Xiao M, Zhang W, Lu C (2015) A super biosorbent from dendrimer poly(amidoamine)-grafted cellulose nanofibril aerogels for effective removal of Cr(vi). J Mater Chem A 3(28):14703–14711
Xu Y, Zhao D (2005) Removal of copper from contaminated soil by use of poly (amidoamine) dendrimers. Environ Sci Technol 39(7):2369–2375
Diallo MS, Christie S, Swaminathan P, Johnson JH, Goddard WA (2005) Dendrimer enhanced ultrafiltration. 1. Recovery of Cu (II) from aqueous solutions using PAMAM dendrimers with ethylene diamine core and terminal NH2 groups. Environ Sci Technol 39(5):1366–1377
Wang P, Lo IM (2009) Synthesis of mesoporous magnetic gamma-Fe2O3 and its application to Cr(VI) removal from contaminated water. Water Res 43(15):3727–3734
Badruddoza AZ, Shawon ZB, Tay WJ, Hidajat K, Uddin MS (2013) Fe3O4/cyclodextrin polymer nanocomposites for selective heavy metals removal from industrial wastewater. Carbohydr Polym 91(1):322–332
Pang Y, Zeng G, Tang L, Zhang Y, Liu Y, Lei X et al (2011) PEI-grafted magnetic porous powder for highly effective adsorption of heavy metal ions. Desalination 281:278–284
Hao YM, Man C, Hu ZB (2010) Effective removal of Cu(II) ions from aqueous solution by amino-functionalized magnetic nanoparticles. J Hazard Mater 184(1–3):392–399
Vereš J, Orolínová Z (2009) Study of the treated and magnetically modified bentonite as possible sorbents of heavy metals. Acta Montan Slovaca 14(2):152–155
Kainz QM, Reiser O (2014) Polymer-and dendrimer-coated magnetic nanoparticles as versatile supports for catalysts, scavengers, and reagents. Acc Chem Res 47(2):667–677
Chou C-M, Lien H-L (2010) Dendrimer-conjugated magnetic nanoparticles for removal of zinc (II) from aqueous solutions. J Nanopart Res 13(5):2099–2107
Kim L-J, Jang J-W, Park J-W (2014) Nano TiO2-functionalized magnetic-cored dendrimer as a photocatalyst. Appl Catal B 147:973–979
Banerjee SS, Chen DH (2007) Fast removal of copper ions by gum arabic modified magnetic nano-adsorbent. J Hazard Mater 147(3):792–799
Anirudhan TS, Suchithra PS (2008) Synthesis and characterization of tannin-immobilized hydrotalcite as a potential adsorbent of heavy metal ions in effluent treatments. Appl Clay Sci 42(1–2):214–223
Wang J, Zheng S, Shao Y, Liu J, Xu Z, Zhu D (2010) Amino-functionalized Fe(3)O(4)@SiO(2) core-shell magnetic nanomaterial as a novel adsorbent for aqueous heavy metals removal. J Colloid Interface Sci 349(1):293–299
Tang SC, Lo IM (2013) Magnetic nanoparticles: essential factors for sustainable environmental applications. Water Res 47(8):2613–2632
Singh S, Barick KC, Bahadur D (2011) Surface engineered magnetic nanoparticles for removal of toxic metal ions and bacterial pathogens. J Hazard Mater 192(3):1539–1547
Shahbazi A, Younesi H, Badiei A (2011) Functionalized SBA-15 mesoporous silica by melamine-based dendrimer amines for adsorptive characteristics of Pb(II), Cu(II) and Cd(II) heavy metal ions in batch and fixed bed column. Chem Eng J 168(2):505–518
Pan B, Cui D, Sheng Y, Ozkan C, Gao F, He R et al (2007) Dendrimer-modified magnetic nanoparticles enhance efficiency of gene delivery system. Cancer Res 67(17):8156–8163
Yan H, Zhang J, You C, Song Z, Yu B, Shen Y (2009) Influences of different synthesis conditions on properties of Fe3O4 nanoparticles. Mater Chem Phys 113(1):46–52
Petcharoen K, Sirivat A (2012) Synthesis and characterization of magnetite nanoparticles via the chemical co-precipitation method. Mater Sci Eng B 177(5):421–427
Wu S, Sun A, Zhai F, Wang J, Xu W, Zhang Q et al (2011) Fe3O4 magnetic nanoparticles synthesis from tailings by ultrasonic chemical co-precipitation. Mater Lett 65(12):1882–1884
Chandra S, Patel MD, Lang H, Bahadur D (2015) Dendrimer-functionalized magnetic nanoparticles: a new electrode material for electrochemical energy storage devices. J Power Sources 280:217–226
Wang T, Yang W-L, Hong Y, Hou Y-L (2016) Magnetic nanoparticles grafted with amino-riched dendrimer as magnetic flocculant for efficient harvesting of oleaginous microalgae. Chem Eng J 297:304–314
Tavallali H, Deilamy-Rad G, Peykarimah P (2013) Preconcentration and speciation of Cr(III) and Cr(VI) in water and soil samples by spectrometric detection via use of nanosized alumina-coated magnetite solid phase. Environ Monit Assess 185(9):7723–7738
Yu BY, Kwak S-Y (2010) Assembly of magnetite nanocrystals into spherical mesoporous aggregates with a 3-D wormhole-like pore structure. J Mater Chem 20(38):8320
Shebanova ON, Lazor P (2003) Raman spectroscopic study of magnetite (FeFe2O4): a new assignment for the vibrational spectrum. J Solid State Chem 174(2):424–430
Kim HR, Jang JW, Park JW (2016) Carboxymethyl chitosan-modified magnetic-cored dendrimer as an amphoteric adsorbent. J Hazard Mater 317:608–616
Pilapong C, Raiputta C, Chaisupa J, Sittichai S, Thongtem S, Thongtem T (2015) Magnetic-EpCAM nanoprobe as a new platform for efficient targeting, isolating and imaging hepatocellular carcinoma. RSC Adv 5(39):30687–30693
Griffete N, Herbst F, Pinson J, Ammar S, Mangeney C (2011) Preparation of water-soluble magnetic nanocrystals using aryl diazonium salt chemistry. J Am Chem Soc 133(6):1646–1649
Osaka T, Matsunaga T, Nakanishi T, Arakaki A, Niwa D, Iida H (2006) Synthesis of magnetic nanoparticles and their application to bioassays. Anal Bioanal Chem 384(3):593–600
Larrubia MA, Ramis G, Busca G (2000) An FT-IR study of the adsorption of urea and ammonia over V2O5–MoO3–TiO2 SCR catalysts. Appl Catal B 27(3):L145–L151
Ma M, Zhang Y, Yu W, H-y Shen, Zhang H-q GuN (2003) Preparation and characterization of magnetite nanoparticles coated by amino silane. Colloids Surf A 212(2–3):219–226
Cao H, He J, Deng L, Gao X (2009) Fabrication of cyclodextrin-functionalized superparamagnetic Fe3O4/amino-silane core–shell nanoparticles via layer-by-layer method. Appl Surf Sci 255(18):7974–7980
Čampelj S, Makovec D, Drofenik M (2009) Functionalization of magnetic nanoparticles with 3-aminopropyl silane. J Magn Magn Mater 321(10):1346–1350
Lei Z, Li Y, Wei X (2008) A facile two-step modifying process for preparation of poly(SStNa)-grafted Fe3O4/SiO2 particles. J Solid State Chem 181(3):480–486
Wang L, Duan G, Chen S-M, Liu X (2015) Particle size and dispersity control by means of gelatin for high-yield mesoporous silica nanospheres. Ind Eng Chem Res 54(50):12580–12586
Popovic J, Hasegawa G, Moudrakovski I, Maier J (2016) Infiltrated porous oxide monoliths as high lithium transference number electrolytes. J Mater Chem A 4(19):7135–7140
Ko J, Lee J, Yoo B, Ryu J, Sohn D (2016) Capillarity-induced selective ex situ synthesis of metal–organic framework inside mesoporous nanotubes. Microporous Mesoporous Mater 220:16–20
Wang S, Wang K, Dai C, Shi H, Li J (2015) Adsorption of Pb2+on amino-functionalized core–shell magnetic mesoporous SBA-15 silica composite. Chem Eng J 262:897–903
Ahmed MA, Ali SM, El-Dek SI, Galal A (2013) Magnetite–hematite nanoparticles prepared by green methods for heavy metal ions removal from water. Mater Sci Eng B 178(10):744–751
Yu J-X, Wang L-Y, Chi R-A, Zhang Y-F, Xu Z-G, Guo J (2013) Competitive adsorption of Pb2+ and Cd2+ on magnetic modified sugarcane bagasse prepared by two simple steps. Appl Surf Sci 268:163–170
Gan T, Wu K (2008) Sorption of Pb(II) using hydrogen peroxide functionalized activated carbon. Colloids Surf A 330(2–3):91–95
Zhang F, Wang B, He S, Man R (2014) Preparation of graphene-oxide/polyamidoamine dendrimers and their adsorption properties toward some heavy metal ions. J Chem Eng Data 59(5):1719–1726
Bai L, Hu H, Fu W, Wan J, Cheng X, Zhuge L et al (2011) Synthesis of a novel silica-supported dithiocarbamate adsorbent and its properties for the removal of heavy metal ions. J Hazard Mater 195:261–275
Taty-Costodes VC, Fauduet H, Porte C, Delacroix A (2003) Removal of Cd(II) and Pb(II) ions, from aqueous solutions, by adsorption onto sawdust of Pinus sylvestris. J Hazard Mater 105(1–3):121–142
Orolínovaá Z, Mockovčiaková A, Škvarla J (2012) Sorption of cadmium (II) from aqueous solution by magnetic clay composite. Desalination Water Treat 24(1–3):284–292
Alsohaimi IH, Wabaidur SM, Kumar M, Khan MA, Alothman ZA, Abdalla MA (2015) Synthesis, characterization of PMDA/TMSPEDA hybrid nano-composite and its applications as an adsorbent for the removal of bivalent heavy metals ions. Chem Eng J 270:9–21
Azouaou N, Sadaoui Z, Djaafri A, Mokaddem H (2010) Adsorption of cadmium from aqueous solution onto untreated coffee grounds: equilibrium, kinetics and thermodynamics. J Hazard Mater 184(1–3):126–134
Foo KY, Hameed BH (2010) Insights into the modeling of adsorption isotherm systems. Chem Eng J 156(1):2–10
Yakout AA, El-Sokkary RH, Shreadah MA, Abdel Hamid OG (2016) Removal of Cd(II) and Pb(II) from wastewater by using triethylenetetramine functionalized grafted cellulose acetate-manganese dioxide composite. Carbohydr Polym 148:406–414
Benhima H, Chiban M, Sinan F, Seta P, Persin M (2008) Removal of lead and cadmium ions from aqueous solution by adsorption onto micro-particles of dry plants. Colloids Surf B Biointerfaces 61(1):10–16
Zhu C, Liu F, Xu C, Gao J, Chen D, Li A (2015) Enhanced removal of Cu(II) and Ni(II) from saline solution by novel dual-primary-amine chelating resin based on anion-synergism. J Hazard Mater 287C:234–242
Park JH, Ok YS, Kim SH, Cho JS, Heo JS, Delaune RD et al (2016) Competitive adsorption of heavy metals onto sesame straw biochar in aqueous solutions. Chemosphere 142:77–83
Prasher SO, Beaugeard M, Hawari J, Bera P, Patel RM, Kim SH (2004) Biosorption of heavy metals by red algae (Palmaria palmata). Environ Technol 25(10):1097–1106
Misono M, Ei Ochiai, Saito Y, Yoneda Y (1967) A new dual parameter scale for the strength of Lewis acids and bases with the evaluation of their softness. J Inorg Nucl Chem 29(11):2685–2691
Acknowledgements
This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2015R1A2A1A09005838) and by the Republic of Korea Ministry of Environment as the Green Remediation Research Center for Organic–inorganic Combined Contamination (The GAIA Project-2012000550001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, KJ., Park, JW. Stability and reusability of amine-functionalized magnetic-cored dendrimer for heavy metal adsorption. J Mater Sci 52, 843–857 (2017). https://doi.org/10.1007/s10853-016-0380-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-016-0380-z