Abstract
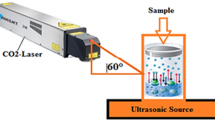
Reduction of graphene oxide (GO) by femtosecond laser pulse irradiation of an aqueous suspension was studied. Different laser parameters such as laser fluence and irradiation time were scanned to obtain the optimum reduced graphene oxide (rGO) with fewer defect sites and lower electrical resistivity. The fabricated rGO samples were characterized using several techniques such as X-ray diffraction, UV–Visible absorption spectrometry, Raman spectroscopy, X-ray photoelectron spectroscopy, and others. The XRD profiles of rGO revealed that the interplanar spacing between carbon layers significantly decreased to 3.51 Å, which is close to that of pristine graphite. Furthermore, the intensity ratio of D and G bands of rGO measured by Raman spectroscopy was more than 20 % smaller than that of GO, indicating the enhancement of sp2 domains. It is noted that the defect sites and the disorder carbon double bond networks on the basal graphene plane were relatively decreased after reduction. In addition, the electrical resistivity of rGO significantly decreased to 3.3 Ω·cm under the optimum condition. From these results, femtosecond laser can be used as a suitable tool for GO reduction because it is a simple, controllable, and flexible method for getting highly reduced graphene oxide.








Similar content being viewed by others
References
Novoselov KS, Geim AK, Morozov SV, Jiang D, Dubonos SV, Grigorieva IV et al (2004) Electric field effect in atomically thin carbon films. Science 306:666–669
Orlita M, Faugeras C, Plochocka P, Neugebauer P, Martinez G, Maude DK et al (2008) Approaching the dirac point in high-mobility multilayer epitaxial graphene. Phys Rev Lett 101:267601–267604
Balandin AA, Gosh S, Bao W, Calizo I, Teweldebrhan D, Miao F et al (2008) Superior thermal conductivity of single-layer graphene. Nano Lett 8:902–907
Lee C, Wei X, Kysar JW, Hone J (2008) Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 321:385–388
Loh KP, Bao Q, Eda G, Chhowalla M (2010) Graphene oxide as a chemically tunable platform for optical application. Nat Chem 2:1015–1024
Zhu Y, Murali S, Stoller MD, Ganesh KJ, Cai W, Ferreira PJ et al (2011) Carbon-based supercapacitors produced by activation of graphene. Science 332:1537–1541
Bian X, Song ZL, Qian Y, Gao W, Cheng ZQ, Chen L et al (2014) Fabrication of graphene-isolated-Au-nanocrystal nanostructures for multimodal cell imaging and photothermal-enhanced chemotherapy. Sci Rep 4:6093–6101
Wang X, Zhi L, Müllen K (2008) Transparent, conductive graphene electrodes for dye-sensitized solar cells. Nano Lett 8:323–327
Hummers WS, Offeman EE (1953) Preparation of graphitic oxide. J Am Chem Soc 80:1339
Eda G, Mattevi C, Yamaguchi H, Kim H, Chhowalla M (2009) Insulator to semimetal transition in graphene oxide. J Phys Chem C 113:15768–15771
Eda G, Franchini G, Chhowalla M (2008) Large-area ultrathin films of reduced graphene oxide as a transparent and flexible electronic material. Nat Nano 3:270–274
Pei S, Cheng H-M (2012) The reduction of graphene oxide. Carbon 50:3210–3228
Cote LJ, Silva RC, Huang J (2009) Flash reduction and patterning of graphite oxide and its polymer composite. J Am Chem Soc 131:11027–11032
Ng YH, Iwase A, Kudo A, Amal R (2010) Reducing graphene oxide on a visible-light BiVO4 photocatalyst for enhanced photoelectrochemical water splitting. J Phys Chem Lett 1:2607–2612
Zhang Y, Guo L, Wei S, He Y, Xia H, Chen Q et al (2010) Direct imprinting of microcircuits on graphene oxides film by femtosecond laser reduction. Nano Today 5:15–20
Compagnini G, Russo P, Tomarchio F, Puglisi O, D’Urso L et al (2012) Laser assisted green synthesis of free standing reduced graphene oxides at the water-air interface. Nanotechnology 23:505601–505606
Gao W, Singh N, Song L, Liu Z, Reddy ALM, Ci L et al (2011) Direct laser writing of micro-supercapacitors on hydrated graphite oxide films. Nat Nanotechnol. doi:10.1038/NNANO.2011.110
Chichkov BN, Momma S, Nolte S, von Alvensleben F, Tünnermann A (1996) Femtosecond, picosecond and nanosecond laser ablation of solids. Appl Phys A 63:109–115
Miyamoto Y, Zhang H, Tománek D (2010) Photoexfoliation of graphene from graphite: an ab initio study. Phys Rev Lett 104:208302–208305
Zhang H, Miyamoto Y (2012) Graphene production by laser shot on graphene oxide; ab initio prediction. Phys Rev B 85:033402–033405
Marcano DC, Kosynkin DV, Berlin JM, Sinitskii A, Sun Z, Slesarev A et al (2010) Improved synthesis of graphene oxide. ACS Nano 4:4806–4814
Shang J, Ma L, Li J, Ai W, Yu T, Gurzadyan GG (2012) The origin of fluorescence from graphene oxide. Sci Rep 2:792–799
Zhang J, Yang H, Shen G, Cheng P, Zhang J, Guo S (2010) Reduction of graphene oxide vial-ascorbic acid. Chem Commun 46:1112–1114
Spanò SF, Isgrò G, Russo P, Fragalà ME, Compagnini G (2014) Tunable properties of graphene oxide reduced by laser irradiation. Appl Phys A 117:19–23
Nethravathi C, Rajamathi M (2008) Chemical modified graphene sheets produced by the solvothermal reduction of colloidal dispersions of graphite oxide. Carbon 46:1994–1998
Kim MC, Hwang GS, Ruoff RS (2009) Epoxide reduction with hydrazine on graphene: a first principles study. J Chem Phys 131:064704–064708
Shin HJ, Kim KK, Benayad A, Yoon SM, Park HK, Jung IS et al (2009) Efficient reduction of graphite oxide by sodium borohydride and its effect on electrical conductance. Adv Funct Mater 19:1987–1992
Lerf A, He H, Forster M, Klinowski J (1998) Structure of graphite oxide revisited. J Phys Chem B 102:4477–4482
Whitby RLD, Gun’ko VM, Korobeinyk A, Busquets R, Cundy AB, Lászlo K (2012) Driving forces of conformational changes in single-layer graphene oxide. ACS Nano 6:3967–3973
Yan J, Zhang Y, Kim P, Pinczuk A (2007) Electric field effect tuning of electron-phonon coupling in graphene. Phys Rev Lett 98:166802–166805
Ferrari AC, Robertson J (2000) Interpretation of Raman spectra of disordered and amorphous carbon. Phys Rev B 61:14095–14107
Valipour A, Hamnabard N, Ahn YH (2015) Performance evaluation of highly conductive graphene (RGOHI-AcOH) and graphene/metal nanoparticles composite (RGO/Ni) coated on carbon cloth for supercapacitor application. RSC Adv 5:92970–92979
Chen W, Yan L, Bangal PR (2010) Preparation of graphene by the rapid and mild thermal reduction of graphene oxide induced by microwaves. Carbon 48:1146–1152
Ji T, Hua Y, Sun M, Ma N (2013) The mechanism of the reaction of graphite oxide to reduced graphene oxide under ultraviolet irradiation. Carbon 54:412–418
Nipane SV, Mali MG, Gokavi GS (2014) Reduced graphene oxide supported silicotungstic acid for efficient conversion of thiols to disulfides by hydrogen peroxide. Ind Eng Chem Res 53:3924–3930
Pommeret S, Gobert F, Mostafavi M, Lampre I, Mialocq JC (2001) Femtochemistry of the hydrated electron at decimolar concentration. J Phys Chem A 105:11400–11406
Xu SC, Irle S, Musaev DG, Lin MC (2006) Quantum chemical study of the dissociative adsorption of OH and H2O on pristine and defective graphite (0001) surfaces: reaction mechanisms and kinetics. J Phys Chem C 111:1355–1365
Gao X, Jang J, Nagase S (2009) Hydrazine and thermal reduction of graphene oxide: reaction mechanisms, product structures, and reaction design. J Phys Chem C 114:832–842
Chin SL, Lagacé S (1996) Generation of H2, O2 and H2O from water by the use of intense femtosecond laser pulses and the possibility of laser sterilization. Appl Opt 35:907–911
Besner S, Meunir M (2010) Femtosecond laser synthesis of Au Ag nanoalloys: photoinduced oxidation and ions release. J Phys Chem C 114:10403–10409
Xing WL, Lalwani G, Rusakova I, Sitharaman B (2014) Degradation of graphene by hydrogen peroxide. Part Part Syst Char 31:745–750
Wittmann G, Horváth I, Dombi A (2002) UV-induced decomposition of ozone and hydrogen peroxide in the aqueous phase at pH 2–7. Ozone Sci Eng 24:281–291
Liu B, Yin S, Wang Y, Guo C, Wu X, Dong Q et al (2015) A facile one-step solvothermal synthesis and electrical properties of reduced graphene oxide/rod-shaped potassium tungsten bronze nanocomposite. J Nanosci Nanotechnol 15:7305–7310
Acknowledgements
The authors would like to acknowledge Mr. Yoshihiro Ojiro, Dr. Shuichi Ogawa, and Prof. Yuji Takakuwa for measurement of electrical resistibility of GO and rGO using a four-point probe method. The first author is financially supported by Indonesia Endowment Fund for Education (LPDP).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that there is no conflict of interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Muttaqin, Nakamura, T., Nishina, Y. et al. Chemical surface modification of graphene oxide by femtosecond laser pulse irradiation in aqueous suspensions. J Mater Sci 52, 749–759 (2017). https://doi.org/10.1007/s10853-016-0368-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-016-0368-8