Abstract
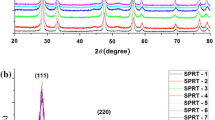
High-purity-doped ceria compounds Ce1-x RE x O2-δ (RE = Sm, Gd; 0 ≤ x ≤ 0.30) were synthesized by the Pechini method at 400 °C. Nanostructured products of crystallite size 5–11 nm were obtained, presenting a single fluorite cubic phase and high surface area values in the range of 75–110 m2g−1. Dense ceramic products with a relative density of 96–98 % resulted after sintering at 1450 °C. Impedance spectroscopy was used to study the conductivity of these compounds in the temperature range of 200–750 °C. Between 600 and 730 °C, the compounds with x = 0.15, 0.20 show conductivities on the order of 10−1 Scm−1, with activation energies between 0.9 and 1.1 eV. Surface areas and conductivity values were found to be comparable with the highest values reported in the literature for similar compounds.








Similar content being viewed by others
References
Steele BCH, Heinzel A (2001) Materials for fuel-cell technologies. Nature 414:345–352. doi:10.1038/35104620
Haile SM (2003) Fuel cell materials and components. Acta Mater 51:5981–6000. doi:10.1016/j.actamat.2003.08.004
Dokiya Masayuki (2002) SOFC system and technology. Solid State Ionics 152–153:383–392. doi:10.1016/S0167-2738(02)00345-4
Neelima M, Amitava B, Alka G, Shobit O, Kantesh B (2015) Progress in material selection for solid oxide fuel cell technology: a review. Prog Mater Sci 72:141–337. doi:10.1016/j.pmatsci.2015.01.001
O’Hayre R, Cha SW, Colella W, Prinz FB (2009) Fuel cell fundamentals, 2nd edn. Willey, New York. ISBN 978-0-470-25843-9
Shao Z, Zhou W, Zhu Z (2012) Advanced synthesis of materials for intermediate-temperature solid oxide fuel cells. Prog Mater Sci 57:804–874. doi:10.1016/j.pmatsci.2011.08.002
Fergus JW (2006) Electrolytes for solid oxide fuel cells. J Power Sources 162:30–40. doi:10.1016/j.jpowsour.2006.06.062
Jaiswal N, Gupta B, Kumar D, Parkash O (2015) Effect of addition of erbium stabilized bismuth oxide on the conductivity of lanthanum doped ceria solid electrolyte for IT-SOFCs. J Alloys Compd 633:174–182. doi:10.1016/j.jallcom.2014.12.243
Hirano M, Inagaki M, Mizutani Y, Nomura K, Kawai M, Nakamura Y (2000) Mechanical and electrical properties of Sc2O3-doped zirconia ceramics improved by post sintering with HIP. Solid State Ionics 133:1–9. doi:10.1016/S0167-2738(00)00706-2
Mizutani Y, Tamura M, Kawai M, Yamamoto O (1994) Development of high-performance electrolyte in SOFC. Solid State Ionics 72(2):271–275. doi:10.1016/0167-2738(94)90158-9
Tietz F (1999) Thermal expansion of SOFC materials. Ionics 5:129–139. doi:10.1007/BF02375916
Muccillo R, Buissa Netto RC, Muccillo ENS (2001) Synthesis and characterization of calcia fully stabilized zirconia solid electrolytes. Mater Lett 49(3–4):197–201. doi:10.1016/S0167-577X(00)00367-0
Stevenson JW, Hasinska K, Canfield NL, Armstrong TR (2000) Influence of cobalt and iron additions on the electrical and thermal properties of (La, Sr)(Ga, Mg)O3−δ . J Electrochem Soc 147(9):3213–3218. doi:10.1149/1.1393885
Lybye D, Poulsen FW, Mogensen M (2000) Conductivity of A- and B-site doped LaAlO3, LaGaO3, LaScO3 and LaInO3 perovskites. Solid State Ionics 128:91–103. doi:10.1016/S0167-2738(99)00337-9
Nguyen TL, Dokiya M (2000) Electrical conductivity, thermal expansion and reaction of (La, Sr)(Ga, Mg)O3 and (La, Sr)AlO3 system. Solid State Ionics 132:217–226. doi:10.1016/S0167-2738(00)00661-5
Zhang TS, Ma J, Cheng H, Chan SH (2006) Ionic conductivity of high-purity Gd-doped ceria solid solutions. Mater Res Bull 41(3):563–568. doi:10.1016/j.materresbull.2005.09.008
Kharton VV, Figueiredo FM, Navarro L, Naumovich EN, Kovalevsky AV, Yaremchenko AA, Viskup AP, Carneiro A, Marques FMB, Frade JR (2001) Ceria-based materials for solid oxide fuel cells. J Mater Sci 36(5):1105–1117. doi:10.1023/A:1004817506146
Mogensen M, Lindegaard T, Hansen UR, Mogensen G (1994) Physical properties of mixed conductor solid oxide fuel cell anodes of doped CeO2. J Electrochem Soc 141(8):2122–2128. doi:10.1149/1.2055072
Herle JV, Horita T, Kawada T, Sakai N, Yokokawa H, Dokiya M (1996) Low temperature fabrication of (Y, Gd, Sm)-doped ceria electrolyte. Solid State Ionics 86–88:1255–1258. doi:10.1016/0167-2738(96)00297-4
Xu H, Yan H, Chen Z (2006) Sintering and electrical properties of Ce0.8Y0.2O1.9 powders prepared by citric acid-nitrate low temperature combustion process. J Power Sources 163(1):409–414. doi:10.1016/j.jpowsour.2006.09.021
Zhu B (1999) Fast ionic conducting film ceramic membranes with advanced applications. Solid State Ionics 119:305–310. doi:10.1016/S0167-2738(98)00519-0
Gerhardt-Anderson R, Nowick AS (1981) Ionic conductivity of CeO2 with trivalent dopants of different ionic radii. Solid State Ionics 5:547–550. doi:10.1016/0167-2738(81)90313-1
Kilner JA (1983) Fast anion transport in solids. Solid State Ionics 8:201–207. doi:10.1016/0167-2738(83)90017-6
Eguchi K, Setoguchi T, Inoue HA (1992) Electrical properties of ceria-based oxides and their application to solid oxide fuel cells. Solid State Ionics 52:165–172. doi:10.1016/0167-2738(92)90102-U
Keshmiri M, Kesler O (2006) Colloidal formation of monodisperse YSZ spheres: kinetics of nucleation and growth. Acta Mater 54(16):4149–4157. doi:10.1016/j.actamat.2006.05.010
Nualpang W, Laosiripojana N, Assabumrungrat S, Injarean U, Pichestapong P, Charojrochkul S (2008) Combustion synthesis of Ce0.9Gd0.1O1.95 for use as an electrolyte for SOFCs. J Metals Mater Miner 18(2):223–227. doi: 10.1016/j.jallcom.2012.06.036. http://www.material.chula.ac.th/Journal/v18-2-2/223-227%20NUALPANG.pdf
Terribile D, Trovarelli A, Leitenburg C, Dolcetti G (1997) Unusual oxygen storage/redox behavior of high-surface-area ceria prepared by a surfactant-assisted route. J Chem Mater 9:2676–2678. doi:10.1021/cm9702732
Ishihara T, Kilner JA, Honda M, Sakai N, Yokokawa H, Takita Y (1998) Oxygen surface exchange and diffusion in LaGaO3 based perovskite type oxides. Solid State Ionics 113:593–600. doi:10.1016/S0167-2738(98)00390-7
Kaneko K, Inoke K, Freitag B, Hungria AB, Midgley Paul A, Hansen TW, Zhang J, Ohara S, Adschiri T (2007) Structural and morphological characterization of cerium oxide nanocrystals prepared by hydrothermal synthesis. Nano Lett 7(2):421–425. doi:10.1021/nl062677b
Prado-Gonjal J, Schmidt R, Espindola-Canuto J, Ramos-Alvarez P, Moran E (2012) Increased ionic conductivity in microwave hydrothermally synthesized rare-earth doped ceria Ce1-x RE x O2-(x/2). J Power Sources 209:163–171. doi:10.1016/j.jpowsour.2012.02.082
Song X, Jiang N, Li Y, Xu D, Qiu G (2007) Synthesis and Characterization of Y-doped mesoporous CeO2 using a chemical precipitation method. J Rare Earths 25:428–433. doi:10.1016/S1002-0721(07)60450-5
Lapa CM, Figueiredo FM, De Souza DPF, Song L, Zhu B, Marques FMB (2010) Synthesis and characterization of composite electrolytes based on samaria-doped ceria and Na/Li carbonates. Int J Hydrog Energy 35:2953–2957. doi:10.1016/j.ijhydene.2009.05.036
Pérez-Coll D, Núñez P, Frade JR, Abrantes JCC (2003) Conductivity of CGO and CSO ceramics obtained from freeze-dried precursors. Electrochim Acta 48(11):1551–1557. doi:10.1016/S0013-4686(03)00027-6
Laberty-Robert C, Long JW, Lucas EM, Pettigrew KA, Stroud RM, Doescher MS, Rolison DR (2006) Sol–Gel-derived ceria nanoarchitectures: synthesis, characterization, and electrical properties. Chem Mater 18(1):50–58. doi:10.1021/cm051385t
Fuentes RO, Baker RT (2008) Synthesis and properties of Gadolinium-doped ceria solid solutions for IT-SOFC electrolytes. Int J Hydrog Energy 33:3480–3484. doi:10.1016/j.ijhydene.2007.10.026
Varez A, Garcia Gonzalez E, Sanz JJ (2006) Cation miscibility in CeO2–ZrO2 Oxides with fluorite structure A combined TEM, SAED and XRD Rietveld analysis. Mater Chem 16:4249–4256. doi:10.1039/B607778A
Shannon RD (1976) Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst A32:751–767. doi:10.1107/S0567739476001551
Mikrajuddin A, Khairurrijal (2008) Derivation of Scherrer relation using an approach in basic physics course. J Nano Saintek 1(1):28–32. ISSN: 1979-0880
Gonzalez G, Braham C, Lebrun JL, Chastel Y, Seiler W, Figueroa IA (2012) Microstructure and texture of Al2Si x Sn (x = 0, 4, 8%) alloys processed by equal channel angular pressing. Mater Trans 53(7):1234–1239. doi:10.2320/matertrans.M2012011
Arabacıa A, Öksüzömer MF (2012) Preparation and characterization of 10 mol% Gd doped CeO2 (GDC) electrolyte for SOFC applications. Ceram Int 38:6509–6515. doi:10.1016/j.ceramint.2012.05.030
Mercadelli E, Ghetti G, Sanson A, Bonelli R, Albonetti S (2013) Synthesis of CeO2 nano–aggregates of complex morphology. Ceram Int 39:629–634. doi:10.1016/j.ceramint.2012.06.074
Rouquerol J, Avnir D, Fairbridge CW, Everett DH, Haynes JM, Pernicone N, Ramsay JDF, Sing KSW, Unge KK (1994) Recommendations for the characterization of porous solids. Pure Appl Chem 66(8):1739–1758. doi:10.1351/pac199466081739
Mahata T, Das G, Mishra RK, Sharma BP (2005) Combustion synthesis of gadolinia doped ceria powder. J Alloys Compd 391:129–135. doi:10.1016/j.jallcom.2004.07.085
Matovic B, Boškovic S, Lj Zivkovic, Vlajic M, Krstic V (2005) Lattice parameters of Gd-doped ceria electrolytes. Mater Sci Forum 494:175–179
Pavasupree S, Suzuki Y, Pivsa-Art S, Yoshikawa S (2005) Preparation and characterization of mesoporous MO2 (M = Ti, Ce, Zr, and Hf) nanopowders by a modified sol–gel method. Ceram Int 31:959–963. doi:10.1016/j.ceramint.2004.10.009
Gil V, Tartaj J, Moure C (2009) Low temperature synthesis and sintering behavior of Gd-doped ceria nanosized powders: comparison between two synthesis procedures. Bol Soc Esp Ceram V 48(2):69–76. http://boletines.secv.es/upload/20090414132803.20094869.pdf
West AR (1985) Solid state chemistry and its applications. Willey, New York, pp 142. ISBN: 978–0–471–90874–6
Hibino T, Hashimoto A, Suzuki M, Yano M, Yoshida SI, Sano M (2002) A solid oxide fuel cell with a novel geometry that eliminates the need for preparing a thin electrolyte film. J Electrochem Soc 149:A195–A200. doi:10.1149/1.1431573
Inaba H, Tagawa H (1996) Ceria-based solid electrolyte. Solid State Ionics 83:1–16. doi:10.1016/0167-2738(95)00229-4
Christie GM, Van Berkel FPF (1996) Microstructure and ionic conductivity relationships in ceria-gadolinia electrolytes. Solid State Ionics 83:17–27. doi:10.1016/0167-27389500155-7
Zhang TS, Ma J, Cheng H, Chan SH (2006) Ionic conductivity of high-purity Gd-doped ceria solid solutions. Mater Res Bull 41:563–568. doi:10.1016/j.materresbull.2005.09.008
Mogensen M, Sammes NM, Thompsett GA (2000) Physical, chemical and electrochemical properties of pure and doped ceria. Solid State Ionics 129:63–94. doi:10.1016/S0167-2738(99)00318-5
Acharya SA, Gaikwad VM, D’Souza SW, Barman SR (2014) Gd/Sm dopant-modified oxidation state and defect generation in nano-ceria. Solid State Ionics 260:21–29. doi:10.1016/j.ssi.2014.03.008
Alvarado-Flores J, Ávalos-Rodríguez L (2013) Materiales para ánodos, cátodos y electrolitos utilizados en celdas de combustible de óxido sólido (SOFC). Rev Mex Fis 59:66–87. ISSN: 0035–001X
Steele BCH (2000) Appraisal of Ce1-y Gd y O2-y/2 electrolytes for IT–SOFC operation at 500°C. Solid State Ionics 129:95–110. doi:10.1016/S0167-2738(99)00319-7
Chen M, Kim BH, Xu Q, Ahn BK, Kang WJ, Huang D (2009) Synthesis and electrical properties of Ce0.8Sm0.2O1.9 ceramics for IT-SOFC electrolytes by urea–combustion technique. Ceram Int 35:1335–1343. doi:10.1016/j.ceramint.2008.06.014
Yao HC, Zhao XL, Chen X, Wang JC, Ge QQ, Wang JS, Li ZJ (2012) Processing and characterization of CoO and Sm2O3 codoped ceria solid solution electrolyte. J Power Sources 205:180–187. doi:10.1016/j.jpowsour.2012.01.076
Dong Y, Hampshire S, Zhou J, Dong X, Lin B, Meng G (2011) Combustion synthesis and characterization of Cu–Sm co-doped CeO2 electrolytes. J Eur Ceram Soc 31:2365–2376. doi:10.1016/j.jeurceramsoc.2011.04.037
Jinping L (2015) Low temperature fabrication of dense gadolinia-doped ceria electrolyte with enhanced electrical conductivity. Electrochim Acta 178:321–328. doi:10.1016/j.electacta.2015.07.182
Kilner JA, Brook RJ (1982) A study of oxygen ion conductivity in doped non-stoichiometric oxides. Solid State Ionics 6:237–252. doi:10.1016/0167-2738(82)90045-5
Schwarz K (2006) Materials design of solid electrolytes. PNAS 103(10):3497. doi:10.1073/pnas.0600327103
Seo DJ, Ryu KO, Park SB, Kim KY, Song RH (2006) Synthesis and properties of Ce1-x Gd x O2-x/2 solid solution prepared by flame spray pyrolysis. Mater Res Bull 41:359–366. doi:10.1016/j.materresbull.2005.08.012
Torrens R, Sammes NM, Tompsett G (2004) Characterization of Pr- and Sm-doped Ce0.8Gd0.2O2–δ . J Electroceram 13(1):683–689. doi:10.1007/s10832-004-5176-x
Verkerk MJ, Keizer K, Burggraaf AJ (1980) High oxygen ion conduction in sintered oxides of the Bi2O3–Er2O3 system. J Appl Electrochem 10:81–90. doi:10.1007/BF00937342
Huang W, Shuk P, Greenblatt M (1997) Properties of sol–gel prepared Ce1-x Sm x 02-x/2 solid electrolytes. Solid State Ionics 100:23–27. doi:10.1016/S0167-2738(97)00309-3
Sanghavi R, Devanathan R, Nandasiri MI, Kuchibhatla S, Kovarik L, Thevuthasan S, Prasad S (2011) Integrated experimental and modeling study of the ionic conductivity of samaria-doped ceria thin films. Solid State Ionics 204:13–19. doi:10.1016/j.ssi.2011.10.007
Thangadurai V, Weppner W (2004) Ce0.8Sm0.2O1.9: characterization of electronic charge carriers and application in limiting current oxygen sensors. Electrochim Acta 49:1577–1585. doi:10.1016/j.electacta.2003.11.019
Lübke S, Wiemhöfer HD (1999) Electronic conductivity of Gd-doped ceria with additional Pr-doping. Solid State Ionics 117:229–243. doi:10.1016/S0167-2738(98)00408-1
Bellino MG, Lamas DG, Walsöe de Reca NE (2006) Enhanced ionic conductivity in nanostructured heavily doped ceria ceramics. Adv Funct Mater 16:107–113. doi:10.1002/adfm.200500186
Acknowledgements
PERA (175598) thanks CONACyT for grant 203342. The authors gratefully thank D. Cabrero, O. Novelo, J. Romero, H. Pfeiffer (all at the IIM), and A. Ponce (CICATA) for technical assistance and acknowledge PAPIIT-UNAM (IN119010) and CONACyT (ECOS-M13P01) projects for financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Ramos-Alvarez, P., Villafuerte-Castrejón, M.E., González, G. et al. Ceria-based electrolytes with high surface area and improved conductivity for intermediate temperature solid oxide fuel cells. J Mater Sci 52, 519–532 (2017). https://doi.org/10.1007/s10853-016-0350-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-016-0350-5