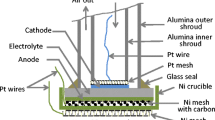
Abstract
Direct carbon fuel cells are an attractive alternative for conventional power generation; however, there is almost no information on the stability of conventional fuel cell materials in direct carbon fuel cell environments, in particular when exposed to realistic fuels and the contaminants contained within these fuels. Similarly, there is little information on the structure and phase assemblage of solid fuels exposed to typical environments found in direct carbon fuel cells. In this paper, we use in situ high-resolution synchrotron powder diffraction on conventional high-temperature fuel cell materials and untreated brown coal to assess the stability and reactivity of these materials at various temperatures up to 850 °C. Materials investigated include nickel metal (Ni), gadolinium-doped ceria (GDC) and yttria-stabilised zirconia (YSZ). The phase stability, crystallite size, lattice parameters and associated linear coefficient of thermal expansion were determined. The phase stability of all fuel cell materials was found to be good with no additional phase formation noted either during heating or after prolonged periods at temperature. An increase in the crystallite size was observed for both GDC and Ni. Non-linear thermal expansion observed in these materials was related to partial reduction of cerium ions (GDC) and due to a Curie point transition (Ni). A wide range of mineral phases were observed in the coal samples and these phases were found to change significantly with temperature. Mineral phases consistent with the ash composition and existing literature on Victorian brown coal were assigned to phases observed.










Similar content being viewed by others
References
Lee C-G, Kim W-K (2015) Oxidation of ash-free coal in a direct carbon fuel cell. Int J Hydrog Energy 40:5475. doi:10.1016/j.ijhydene.2015.01.068
Kaklidis N, Garagounis I, Kyriakou V et al (2015) Direct utilization of lignite coal in a Co–CeO2/YSZ/Ag solid oxide fuel cell. J. Hydrogen Energy, Int. doi:10.1016/j.ijhydene.2015.02.007
Xu K, Chen C, Liu H, Tian Y, Li X, Yao H (2014) Effect of coal based pyrolysis gases on the performance of solid oxide direct carbon fuel cells. Int J Hydrog Energy 39:17845. doi:10.1016/j.ijhydene.2014.08.133
Jiao Y, Zhao J, An W et al (2015) Structurally modified coal char as a fuel for solid oxide-based carbon fuel cells with improved performance. J Power Sources 288:106. doi:10.1016/j.jpowsour.2015.04.121
Rady AC, Giddey S, Kulkarni A, Badwal SPS, Bhattacharya S, Ladewig BP (2014) Direct carbon fuel cell operation on brown coal. Appl Energy 120:56. doi:10.1016/j.apenergy.2014.01.046
Rady AC, Giddey S, Kulkarni A, Badwal SPS, Bhattacharya S (2015) Direct carbon fuel cell operation on brown coal with a Ni-GDC-YSZ anode. Electrochim Acta. doi:10.1016/j.electacta.2015.08.064
Dudek M (2015) On the utilization of coal samples in direct carbon solid oxide fuel cell technology. Solid State Ionics 271:121. doi:10.1016/j.ssi.2014.09.034
Li X, Zhu Z, De Marco R, Bradley J, Dicks A (2010) Evaluation of raw coals as fuels for direct carbon fuel cells. J Power Sources 195:4051. doi:10.1016/j.jpowsour.2010.01.048
Ahn SY, Eom SY, Rhie YH et al (2013) Utilization of wood biomass char in a direct carbon fuel cell (DCFC) system. Appl Energy 105:207. doi:10.1016/j.apenergy.2013.01.023
Kacprzak A, Kobyłecki R, Włodarczyk R, Bis Z (2014) The effect of fuel type on the performance of a direct carbon fuel cell with molten alkaline electrolyte. J Power Sources 255:179. doi:10.1016/j.jpowsour.2014.01.012
Munnings C, Kulkarni A, Giddey S, Badwal SPS (2014) Biomass to power conversion in a direct carbon fuel cell. Int J Hydrog Energy 39:12377. doi:10.1016/j.ijhydene.2014.03.255
Yu J, Zhao Y, Li Y (2014) Utilization of corn cob biochar in a direct carbon fuel cell. J Power Sources 270:312. doi:10.1016/j.jpowsour.2014.07.125
Elleuch A, Halouani K, Li Y (2015) Investigation of chemical and electrochemical reactions mechanisms in a direct carbon fuel cell using olive wood charcoal as sustainable fuel. J Power Sources 281:350. doi:10.1016/j.jpowsour.2015.01.171
Hao W, He X, Mi Y (2014) Achieving high performance in intermediate temperature direct carbon fuel cells with renewable carbon as a fuel source. Appl Energy 135:174. doi:10.1016/j.apenergy.2014.08.055
Bonaccorso AD, Irvine JTS (2012) Development of tubular hybrid direct carbon fuel cell. Int J Hydrog Energy 37:19337. doi:10.1016/j.ijhydene.2012.02.104
Badwal SPS, Fini D, Ciacchi FT, Munnings C, Kimpton JA, Drennan J (2013) Structural and microstructural stability of Ceria-Gadolinia electrolyte exposed to reducing environments of high temperature fuel cells. J Mater Chem A 1:10768. doi:10.1039/c3ta11752a
Steele BCH (2000) Appraisal of Ce1−yGdyO2−y/2 electrolytes for IT-SOFC operation at 500°C. Solid State Ionics 129:95. doi:10.1016/S0167-2738(99)00319-7
Kulkarni A, Giddey S, Badwal SPS (2015) Yttria-doped ceria anode for carbon-fueled solid oxide fuel cell. J Solid State Electrochem 19:325. doi:10.1007/s10008-014-2604-y
Badwal SPS, Ciacchi FT (2000) Oxygen-ion conducting electrolyte materials for solid oxide fuel cells. Ionics 6:1. doi:10.1007/BF02375543
Giddey S, Badwal SPS, Kulkarni A, Munnings C (2012) A comprehensive review of direct carbon fuel cell technology. Progr Energy Combust Sci 38:360. doi:10.1016/j.pecs.2012.01.003
Patterson JW (1971) Conduction domains for solid electrolytes. J Electrochem Soc 118:1033. doi:10.1149/1.2408241
Etsell TH, Flengas SN (1970) Electrical properties of solid oxide electrolytes. Chem Rev (Washington, DC, U. S.) 70:339. doi:10.1021/cr60265a003
Cowin PI, Petit CTG, Lan R, Irvine JTS, Tao S (2011) Recent progress in the development of anode materials for solid oxide fuel cells. Adv Energy Mater 1:314. doi:10.1002/aenm.201100108
Koide H, Someya Y, Yoshida T, Maruyama T (2000) Properties of Ni/YSZ cermet as anode for SOFC. Solid State Ionics 132:253. doi:10.1016/S0167-2738(00)00652-4
Ju H, Eom J, Lee JK et al (2014) Durable power performance of a direct ash-free coal fuel cell. Electrochim Acta. doi:10.1016/j.electacta.2013.10.124
Xu X, Zhou W, Liang F, Zhu Z (2013) A comparative study of different carbon fuels in an electrolyte-supported hybrid direct carbon fuel cell. Appl Energy 108:402. doi:10.1016/j.apenergy.2013.03.053
Gür TM (2013) Critical review of carbon conversion in “carbon fuel cells”. Chem Rev (Washington, DC, U. S.) 113:6179. doi:10.1021/cr400072b
Hibino T, Hashimoto A, Yano M, Suzuki M, Sano M (2003) Ru-catalyzed anode materials for direct hydrocarbon SOFCs. Electrochim Acta 48:2531. doi:10.1016/S0013-4686(03)00296-2
Xu S, Wang X (2005) Highly active and coking resistant Ni/CeO2–ZrO2 catalyst for partial oxidation of methane. Fuel 84:563. doi:10.1016/j.fuel.2004.10.008
Rady AC, Giddey S, Kulkarni A, Badwal SPS, Bhattacharya S (2014) Degradation mechanism in a direct carbon fuel cell operated with demineralised brown coal. Electrochim Acta 143:278. doi:10.1016/j.electacta.2014.07.088
Brockway DJ, Ottrey AL, Higgins RS (1991) The science of victorian brown coal. Butterworth-Heinemann, Oxford
Wallwork KS, Kennedy BJ, Wang D (2007) The high resolution powder diffraction beamline for the Australian synchrotron. AIP Conf Proc. doi:10.1063/1.2436201
Schmitt B, Brönnimann C, Eikenberry EF et al (2003) Mythen detector system. Nucl Instrum Methods Phys Res Sect A 501:267. doi:10.1016/S0168-9002(02)02045-4
Yamamura H, Yagi Y, Kakinuma K (2008) Electrode effects for dielectric properties of 8 mol% Y2O3 stabilized ZrO2. Electrochemistry. http://doi.org/10.5796/electrochemistry.76.734
Swanson HE, Tatge E, S United (1953) Standard X-ray diffraction powder patterns. Vol. I, data for 54 inorganic substances. National Bureanu of Standards, Washington
Li M, Zeng F, Chang H, Xu B, Wang W (2013) Aggregate structure evolution of low-rank coals during pyrolysis by in situ X-ray diffraction. Int J Coal Geol 116–117:262. doi:10.1016/j.coal.2013.07.008
Lu L, Sahajwalla V, Kong C, Harris D (2001) Quantitative X-ray diffraction analysis and its application to various coals. Carbon. doi:10.1016/S0008-6223(00)00318-3
Harold HS (1995) Coal science and technology. Elsevier, Amsterdam
Grim RE (1968) Clay mineralogy. McGraw-Hill, New York
Bakharev T (2006) Thermal behaviour of geopolymers prepared using class F fly ash and elevated temperature curing. Cem Concr Res 36:1134. doi:10.1016/j.cemconres.2006.03.022
Balzar D, Audebrand N, Daymond MR et al (2004) Size–strain line-broadening analysis of the ceria round-robin sample. J Appl Crystallogr 37:911. doi:10.1107/S0021889804022551
Sheldon BW, Mandowara S, Rankin J (2013) Grain boundary induced compositional stress in nanocrystalline ceria films. Solid State Ionics 233:38. doi:10.1016/j.ssi.2012.11.006
Kossoy A, Feldman Y, Korobko R, Wachtel E, Lubomirsky I, Maier J (2009) Influence of point-defect reaction kinetics on the lattice parameter of Ce0.8Gd0.2O1.9. Adv Funct Mater 19:634. doi:10.1002/adfm.200801162
Sheldon BW, Shenoy VB (2011) Space charge induced surface stresses: implications in ceria and other ionic solids. Phys Rev Lett 106:216104. doi:10.1103/PhysRevLett.106.216104
Robinson I, Harder R (2009) Coherent X-ray diffraction imaging of strain at the nanoscale. Nat Mater 8:291. doi:10.1038/nmat2400
Hayashi H, Kanoh M, Quan CJ et al (2000) Thermal expansion of Gd-doped ceria and reduced ceria. Solid State Ionics 132:227. doi:10.1016/S0167-2738(00)00646-9
Kossoy A, Feldman Y, Wachtel E et al (2006) On the origin of the lattice constant anomaly in nanocrystalline ceria. Phys Chem Chem Phys 8:1111. doi:10.1039/B513764K
Wu L, Wiesmann HJ, Moodenbaugh AR et al (2004) Oxidation state and lattice expansion of CeO2−x nanoparticles as a function of particle size. Phys Rev B. doi:10.1103/PhysRevB.69.125415
Sen R, Das S, Das K (2011) Microstructural characterization of nanosized ceria powders by X-ray diffraction analysis. Metall Mater Trans 42A:1409. doi:10.1007/s11661-010-0463-4
Tietz F (1999) Thermal expansion of SOFC materials. Ionics 5:129. doi:10.1007/BF02375916
Biswas M, Kumbhar CS, Gowtam DS (2011) Characterization of nanocrystalline Yttria-stabilized zirconia: an in situ HTXRD study. ISRN Nanotechnol. doi:10.5402/2011/305687
Radovic M, Lara-Curzio E, Trejo RM, Wang H, Porter WD (2008) Thermophysical properties of YSZ and Ni-YSZ as a function of temperature and porosity. Advances in solid oxide fuel cells II: Ceramic engineering and science proceedings. Wiley
Badwal SPS (1995) Grain boundary resistivity in zirconia-based materials: effect of sintering temperatures and impurities. Solid State Ionics 76:67. doi:10.1016/0167-2738(94)00236-L
Legendre B, Sghaier M (2011) Curie temperature of nickel. J Therm Anal Calorim 105:141. doi:10.1007/s10973-011-1448-2
Kollie TG (1977) Measurement of the thermal-expansion coefficient of nickel from 300 to 1000 K and determination of the power-law constants near the Curie temperature. Phys Rev B 16:4872. doi:10.1103/PhysRevB.16.4872
Major J, Mezei F, Nagy E, Sváb E, Tichy G (1971) Thermal expansion coefficient of nickel near the Curie point. Phys Lett A 35:377. doi:10.1016/0375-9601(71)90746-8
Söffge F, Steichele E, Stierstadt K (1977) Thermal expansion anomaly of nickel near the Curie point. Phys Status Solidi (a). doi:10.1002/pssa.2210420226
Hwang J-W (1972) Thermal expansion of nickel and iron, and the influence of nitrogen on the lattice parameter of iron at the curie temperature. Dissertation, University of Missouri-Rolla
Natter H, Schmelzer M, Hempelmann R (1998) Nanocrystalline nickel and nickel-copper alloys: synthesis, characterization, and thermal stability. J Mater Res 13:1186. doi:10.1557/JMR.1998.0169
Liu XD, Zhang HY, Lu K, Hu ZQ (1994) The lattice expansion in nanometre-sized Ni polycrystals. J Phys: Condens Matter 6:L497. doi:10.1088/0953-8984/6/34/001
Syukri T Ban, Ohya Y, Takahashi Y (2003) A simple synthesis of metallic Ni and Ni–Co alloy fine powders from a mixed-metal acetate precursor. Mater Chem Phys 78:645. doi:10.1016/S0254-0584(02)00185-2
Hidnert P (1957) Thermal expansion of some nickel alloys. J Res Natl Bur Stand 58:89
Jordan L, Swanger WH (1930) The properties of pure nickel. Bureau of standards. J Res. doi:10.6028/jres.005.075
Acknowledgments
The authors would like to acknowledge the Australia Synchrotron for beamtime and Brown Coal Innovation Australia (BCIA) for partially funding this research. Special thanks are also given to Dr. Justin Kimpton of the Australian Synchrotron for his assistance with setting up batch processing of the refinements as well as with data collection. We would like to thank Aaron Seeber for reviewing this manuscript prior to submission.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rady, A.C., Munnings, C., Giddey, S. et al. In situ high-temperature powder diffraction studies of solid oxide electrolyte direct carbon fuel cell materials in the presence of brown coal. J Mater Sci 51, 3928–3940 (2016). https://doi.org/10.1007/s10853-015-9712-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-015-9712-7