Abstract
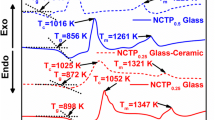
The structural characterization for Na+ Super Ionic Conductor (NASICON)-type glass ceramics of 20R2O–XZrO2–15P2O5–(65-X)SiO2 (R = Na, Li) was investigated by X-ray diffraction (XRD) and X-ray absorption fine structure to clarify the better conductive mechanism of Li-glass ceramics than that of Na-glass ceramics. The Na-glass ceramics were synthesized by heat treatment using glass material, and the Li-glass ceramics were obtained through ion exchange techniques from Na-glass ceramics. From the XRD analysis, the positive correlation between ionic conductivity and lattice parameter was identified for the Na-glass ceramics, on the other hand, there was no positive relation for Li-glass ceramics, indicating that the ion conductive mechanism cannot be explained by the lattice parameter of NASICON crystalline phase alone. The Debye–Waller factors related to the oxygen surrounding the Zr-ion for the Li-glass ceramics were higher than those for the Na-glass ceramics. The static disorder around Zr-ion may cause more weakly bonding between the Li ions and surrounding atoms, resulting in the improvement of ionic conductivity.






Similar content being viewed by others
References
Kamaya N, Homma K, Yamakawa Y, Hirayama M, Kanno R, Yonemura M, Kamiyama T, Kato Y, Hama S, Kawamoto K, Mitsui A (2011) A lithium superionic conductor. Nat Mater 10:682–686
Kanno R, Murayama M (2001) Lithium ionic conductor thio-LISICON: the Li2S–GeS2–P2S5 system. J Electrochem Soc 148:A742–A746
Boulineau S, Courty M, Tarascon JM, Viallet V (2012) Mechanochemical synthesis of Li-argyrodite Li6PS5X (X = Cl, Br, I) as sulfur-based solid electrolytes for all solid state batteries application. Solid State Ionics 221:1–5
Takada K, Aotani N, Iwamoto K, Kondo S (1996) Solid state lithium battery with oxysulfide glass. Solid State Ionics 86–88:877–882
Mizuno F, Hayashi A, Tadanaga K, Tatsumisago M (2006) High lithium ion conducting glass-ceramics in the system Li2S-P2S5. Solid State Ionics 177:2721–2725
Kawakami Y, Ikuta H, Uchida T, Wakihara M (1997) Ionic conduction of lithium in Li2S SiS2 Li4SiO4 glass system. Thermochim Acta 299:7–12
Muramatsu H, Hayashi A, Ohtomo T, Hama S, Tatsumisago M (2011) Structural change of Li2S–P2S5 sulfide solid electrolytes in the atmosphere. Solid State Ionics 182:116–119
Chowdari BVR, Subba Rao GV, Lee GYH (2000) XPS and ionic conductivity studies on Li2O–Al2O3–(TiO2 or GeO2)-P2O5 glass-ceramics. Solid State Ionics 136–137:1067–1075
Fu J (1997) Fast Li+ ion conducting glass-ceramics in the system Li2O-Al2O3-GeO2-P2O5. Solid State Ionics 104:191–194
Fu J (1997) Superionic conductivity of glass-ceramics in the system Li2O Al2O3 TiO2 P2O5. Solid State Ionics 96:195–200
Abrahams I, Hadzifejzovic E (2000) Lithium ion conductivity and thermal behaviour of glasses and crystallised glasses in the system Li2O–Al2O3–TiO2–P2O5. Solid State Ionics 134:249–257
Fu J (1998) Fast Li+ ion conduction in Li2O–(Al2O3 Ga2O3)–TiO2–P2O5 glass-ceramics. J Mater Sci 33:1549–1553. doi:10.1023/A:1017559619391
Katoh T, Inda Y, Baba M, Ye R (2010) Lithium-ion conductive glass-ceramics with composition ratio control and their electrochemical characteristics. J Ceram Soc Jpn 118:159–1162
Shimonishi Y, Zhang T, Imanishi N, Im D, Lee DJ, Hirano A, Takeda Y, Yamamoto O, Sammes N (2011) A study on lithium/air secondary batteries—stability of the NASICON-type lithium ion conducting solid electrolyte in alkaline aqueous solutions. J Power Sources 196:5128–5132
Shimonishi Y, Zhang T, Johnson P, Imanishi N, Hirano A, Takeda Y, Yamamoto O, Sammes N (2010) A study on lithium/air secondary batteries—stability of NASICON-type glass ceramics in acid solutions. J Power Sources 195:6187–6191
Tsujimura T, Koike A, Kuroki Y (2012) Li-ion conductive phosphate glass synthesized by using ion exchange. ECS Trans 45:135–141
Tsujimura T (2014) Li-ion conductive phosphosilicate glass ceramics synthesized by ion exchange. Solid State Ionics 262:829–832
Yao YF, Kummer JT (1967) Ion exchange properties of and rates of ionic diffusion in beta-alumina. J Inorg Nucl Chem 29:2453–2475
Morimoto S (1989) Ionic conductivity of Na2O–ZrO2–P2O5–SiO2 system glass ceramics. J Ceram Soc Jpn 97:1097–1103 (In Japanese with English abstract)
Ravel B, Newville M (2005) ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J Synchrotron Radiat 12:537–541
Bohnke O, Ronchetti S, Mazza D (1999) Conductivity measurements on nasicon and nasicon-modified materials. Solid State Ionics 122:127–136
Nagakane T, Yamauchi H, Yuki K, Ohji M, Sakamoto A, Komatsu T, Honma T, Zou M, Park G, Sakai T (2012) Glass-ceramic LiFePO4 for lithium-ion rechargeable battery. Solid State Ionics 206:78–83
Kageyama H, Kamijo N, Asai T, Saito Y, Ado K, Nakamura O (1990) XAFS study of nasicon-related compounds, Na3Zr0.5Co0.5FeP3O12 and Na3Zr0.5Fe(II)0.5Fe(III)P3O12. Solid State Ionics 40–41:350–356
Okumura T, Ina T, Orikasa Y, Arai H, Uchimoto Y, Ogumi Z (2011) Improvement of lithium ion conductivity for A-site disordered lithium lanthanum titanate perovskite oxides by fluoride ion substitution. J Mater Chem 21:10061–10068
Acknowledgements
The synchrotron radiation experiments were performed at BL14B2 (proposal No. 2013A1784, No. 2013B1832), at BL19B2 (proposal No. 2013B1593) with the approval of the SPring8. The author is grateful to Dr. T. Itoh (AGC Seimi Chemical Co. Ltd.) and Dr. S. Matsumoto (Okayama University) for extending technical support for the synchrotron experiments and discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsujimura, T. Structural characterization for alkali ion conductive phosphosilicate glass ceramics. J Mater Sci 50, 7735–7741 (2015). https://doi.org/10.1007/s10853-015-9342-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-015-9342-0