Abstract
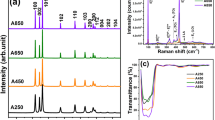
The compositional dependence of the crystalline phase and properties of precipitates (Ti x Sn1−x O2) in the TiO2–SnO2 system, which were hydrothermally formed at 100–200 °C from the precursor solutions of TiCl4 and SnCl4 under weakly basic conditions in the presence of tetramethylammonium hydroxide (TMAH) was investigated. Rutile-type (Ti, Sn)O2 solid solutions with nano-sized crystallite were directly formed at 180 °C in the composition range of x = 0–0.8. Nanoparticles with anatase crystallite around 10 nm as a main crystalline phase of precipitates that were formed in the compositions x = 0.9 and 1.0 showed similar photocatalytic activity. As the hydrothermal treatment temperature rose from 100 to 200 °C, the crystallite size of rutile solid solution, Ti0.5Sn0.5O2, increased from 2.5 to 8.0 nm. The optical band gap of the samples changed in the range of 2.93–3.25 eV depending on their composition in the system. At the composition of x = 1.0, submicron-sized anatase-type pure TiO2 particles (sizes of cuboid sides are around 100–120 nm) with pretty high crystallinity and superior photocatalytic activity were formed from the aqueous solution of TiCl4 under basic hydrothermal condition at 180 °C in the presence of TMAH with concentration as 1.3 times high as the condition in the case of the nano-sized anatase.













Similar content being viewed by others
References
Gordon RG (2000) Criteria for choosing transparent conductors. MRS Bull 25:52–57
Yang T, Qin X, Wang Hh, Jia Q, Yu R, Wang B, Wang J, Ibrahim K, Jiang X, He Q (2010) Preparation and application in p-n homojunction diode of p-type transparent conducting Ga-doped SnO2 thin films. Thin Solid Films 518:5542–5545
Cao L, Spiess FJ, Huang A, Suib SL, Obee TN, Hay SO, Freihaut JD (1999) Heterogeneous photocatalytic oxidation of 1-butene on SnO2 and TiO2 films. J Phys Chem B 103:2912–2917
Prasad RM, Gurlo A, Riedel R, Huebner M, Barsan N, Weimar U (2010) Microporous ceramic coated SnO2 sensors for hydrogen and carbon monoxide sensing in harsh reducing conditions. Sens Actuat B 149:105–109
Radecka M, Zakrzewska K, Rekas M (1998) SnO2–TiO2 solid solutions for gas sensors. Sens Actuat B 47:194–204
Tai WP, Oh JH (2002) Fabrication and humidity sensing properties of nanostructured TiO2–SnO2 thin films. Sens Actuat B 85:154–157
Lin J, Yu JC, Lo D, Lam SK (1999) Photocatalytic activity of rutile Ti1−x Sn x O2 solid solutions. J Catal 183:368–372
Tada H, Hattori A, Tokihisa Y, Imai K, Tohge N, Ito S (2000) A patterned-TiO2/SnO2 bilayer type photocatalyst. J Phys Chem B 104:4585–4587
Fresno F, Coronado JA, Tudela D, Soria J (2005) Influence of the structural characteristics of Ti1-xSnxO2 nanoparticles on their photocatalytic activity for the elimination of methylcyclohexane vapors. Appl Catal B 55:159–167
Cai ZQ, Shen QH, Gao JW, Yang H (2007) Low-temperature preparation of TiO2/SnO2 composite film and its photocatalytic activity. J Inorg Mater 22:733–736
Kutty TRN, Avudaithai M (1989) Photocatalytic activity on tin-substituted titanium dioxide in visible light. Chem Phys Lett 163:93–97
Sayilkan F, Asiltuerk M, Tatar P, Kiraz N, Sener S, Arpac E, Sayilkan H (2008) Photocatalytic performance of Sn-doped TiO2 nanostructured thin films for photocatalytic degradation of malachite green dye under UV and VIS-lights. Mater Res Bull 43:127–134
Fox MA, Dulay MT (1993) Heterogeneous photocatalysis. Chem Rev 93:341–357
Carp O, Huisman CL, Reller A (2004) Photoinduced reactivity of titanium dioxide. Prog Solid State Chem 32:33–177
Bach U, Lupo D, Comte P, Moser JE, Weissortel F, Salbeck J, Spreitzer H, Graetzel M (1998) Solid-state dye-sensitized mesoporous TiO2 solar cells with high photon-to-electron conversion efficiencies. Nature 395:583–585
Park JY, Song SJ, Wachsman ED (2010) Highly sensitive/selective miniature potentiometric carbon monoxide gas sensors with titania-based sensing elements. J Am Ceram Soc 93:1062–1068
Furubayashi Y, Hitosugi T, Yamamoto Y, Inaba K, Kinoda G, Hirose Y, Shimada T, Hasegawa T (2005) A transparent metal: Nb-doped anatase TiO2. Appl Phys Lett 86;252101
Huang SY, Kavan L, Exnar I, Graetzel M (1995) Rocking chair lithium battery based on nanocrystalline TiO2 (anatase). J Electrochem Soc 142:L142–L144
Sinclair WR, Loomis TC (1959) Measurements of diffusion in the system TiO2–SnO2. Kinet High-Temp Processes Conf Dedham Mass 1958:58–61
Drobeck DL, Virkar AV, Cohen RM (1990) 1. The effect of aliovalent dopants on cation diffusion in titanium dioxide–tin dioxide. J Phys Chem Solids 51:977–988
Cushing BL, Kolesnichenko VL, O’Connor CJ (2004) Recent advances in the liquid–phase syntheses of inorganic nanoparticles. Chem Rev 104:3893–3946
Byrappa K, Adschiri T (2007) Hydrothermal technology for nanotechnology. Prog Cryst Growth Charact 53:117–166
Hirano M, Morikawa H, Inagaki M, Toyoda M (2002) Direct synthesis of new zircon-type ZrGeO4 and Zr(Ge, Si)O4 solid solutions. J Am Ceram Soc 85:1915–1920
Kasuga T, Hiramatsu M, Hoson A, Sekino T, Niihara K (1999) Titania nanotubes prepared by chemical processing. Adv Mater 11:1307–1311
Yin AX, Min XQ, Zhang YW, Yan CH (2011) Shape-selective synthesis and facet-dependent enhanced electrocatalytic activity and durability of monodisperse sub-10 nm Pt–Pd tetrahedrons and cubes. J Am Chem Soc 133:3816–3819
Hirano M, Kono T (2011) Hydrothermal synthesis of rutile-type complete solid solution nanoparticles in the TiO2–SnO2 system under acidic conditions. J Am Ceram Soc 94:3319–3326
Hirano M, Kato E (1996) Hydrothermal synthesis and sintering of fine powders in CeO2–ZrO2 system. J Ceram Soc Jpn 104:958–962
Hirano M, Ito T (2008) Direct formation of new, phase-stable, and photoactive anatase-type Ti1−2x Nb x Sc x O2 solid solution nanoparticles by hydrothermal method. Mater Res Bull 43:2196–2206
Hernandez GP, Morales AE, Pal U, Anota EC (2012) Morphology evolution of hydrothermally grown ZnO nanostructures on gallium doping and their defect structures. Mater Chem Phys 135:810–817
Sadhana K, Praveena K, Bharadwaj S, Murthy SR (2009) Microwave-hydrothermal synthesis of BaTiO3+NiCuZnFe2O4 nanocomposites. J Alloys Compd 472:484–488
Hirano M, Dozono H (2013) Luminescence nanocrystals in the rare-earth niobate–zirconia system formed via hydrothermal method. J Solid State Chem 204:335–340
Hirano M, Kono T (2011) Hydrothermal synthesis and properties of solid solutions and composite nanoparticles in the TiO2–SnO2 system. Mater Res Bull 46:13841390
Hirano M, Ota K, Iwata H (2004) Direct formation of anatase (TiO2)/silica (SiO2) composite nanoparticles with high phase stability of 1300 °C from acidic solution by hydrolysis under hydrothermal condition. Chem Mater 16:3725–3732
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hirano, M., Takahashi, M. Submicron-sized anatase, TiO2 with high photocatalytic activity, and (Ti, Sn)O2 nanocrystals formed via hydrothermal technique. J Mater Sci 49, 8163–8170 (2014). https://doi.org/10.1007/s10853-014-8525-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-014-8525-4