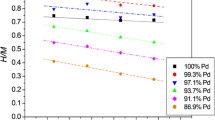
Abstract
Silver–palladium alloys have various industrial applications, such as in hydrogen permeation and hydrogen storage materials. The objective of the present study is to determine experimentally the thermodynamic properties of solid state silver–palladium alloys in low temperatures. Thermodynamic measurements of silver–palladium alloys have been performed over a temperature range of 450–750 K by the electromotive force method with superionic conductor AgI as the solid electrolyte. The activity and partial molar Gibbs energy of silver were obtained for Ag–Pd alloys over the whole composition range, and the thermodynamic properties of palladium were calculated using the Gibbs–Duhem equation. The results show that activities of silver exhibit fairly large negative deviations over most of the composition range, and the activities of palladium are characterized by both negative and positive deviations from the ideal Raoultian behavior. The results also show the minimum integral enthalpy of mixing of Ag–Pd alloys to locate at around 60 at.% Ag.







Similar content being viewed by others
References
Pratt JN (1960) The thermodynamic properties of silver–palladium alloys. Trans Faraday Soc 56:975–987
Lewis F, Magennis J, Mckee S, Ssebuwufu P (1983) Hydrogen chemical-potentials and diffusion-coefficients in hydrogen diffusion membranes. Nature 306:673–675
Picard C, Kleppa O, Boureau G (1979) High-temperature thermodynamics of the solutions of hydrogen in palladium–silver alloys. J Chem Phys 70:2710–2719
Ghosh G, Kantner C, Olson GB (1999) Thermodynamic modeling of the Pd–X (X = Ag Co, Fe, Ni) systems. J Phase Equilibria 20:295–308
Ruer R (1906) Über die Legierungen des Palladiums mit Silber. Z Anorg Chem 51:315–319
Dinsdale AT (1991) SGTE data for pure elements. Calphad 15:317–425
McKeehan LW (1922) The crystal structure of silver–palladium and silver–gold alloys. Phys Rev 20:424–432
Coles B (1956) The lattice spacings of nickel copper and palladium silver alloys. J Inst Metals 84:346–348
Hoare FE, Yates B (1957) The low-temperature (2 to 4.2 K) specific heats of palladium–silver alloys. Proc R Soc Lond A 240:42–53
Raychaudhuri PK (1971) Mass spectrometric and galvanic cell studies of the thermodynamic properties of solid silver–palladium alloy. PhD Dissertation, Northwestern University
Oriani R, Murphy WK (1962) Thermodynamics of ordering alloys: IV heats of formation of some alloys of transition metals. Acta Metall 10:879–885
Chan JP, Hultgren R (1969) The thermodynamic properties of silver+palladium alloys. J Chem Thermodyn 1:45–50
Luef C, Paul A, Flandorfer H, Kodentsov A, Ipser H (2005) Enthalpies of mixing of metallic systems relevant for lead-free soldering: Ag–Pd and Ag–Pd–Sn. J Alloys Compd 391:67–76
Myles KM (1965) Thermodynamic properties of solid palladium–silver alloys. Acta Metall 13:109–113
Eremenko VN, Lukashenko GM, Pritula VL (1968) Investigation of the thermodynamic properties of silver–palladium solid solutions by vapor pressure measurement. Russ J Phys Chem 42:346–347
Schmahl NG (1951) Die Erleichterung der Reduzierbarkeit von Metallverbindungen durch Legierungsbildung und ihre Berechnung. Z Anorg Allg Chem 266:1–29
Savitskii EM, Pravoverov NL (1961) Kurnakov phases in the palladium–silver system. Russ J Inorg Chem 6:253–254
Garino T, Rodriquez M (2000) Behavior of silver and palladium mixtures during heating. J Am Ceram Soc 83:2709–2714
Karakaya I, Thompson WT (1988) The Ag–Pd (silver–palladium) system. Bull Alloy Phase Diagr 9:237–243
Sopoušek J, Zemanová A, Vřešťál J, Brož P (2010) Experimental determination of phase equilibria and reassessment of Ag–Pd system. J Alloys Compd 504:431–434
Feng D, Taskinen P, Tesfaye F (2013) Thermodynamic stability of Ag2Se from 350 to 500 K by a solid state galvanic cell. Solid State Ion 231:1–4
Feng D, Taskinen P (2014) Thermodynamic stability of AuSe at temperature from (400 to 700) K by a solid state galvanic cell. J Chem Thermodyn 71:98–102
Feng D, Taskinen P (2014) Thermodynamic properties of Ag3AuSe2 from 300 to 500 K by a solid state galvanic cell. J Alloys Compd 583:176–179
Darken LS, Gurry RW (1953) Physical chemistry of metals. McGraw-Hill, New York
Wagner C (1952) Thermodynamics of alloys. Addison-Wesley Press, Cambridge
Margules M (1895) Über die Zusammensetzung der gesättigten Dämpfe von Misschungen. Sitzber Akad Wiss Wien 104:1243–1278
Meschel SV, Kleppa OJ (2003) Thermochemistry of some binary alloys of noble metals (Cu, Ag, Au) and transition metals by high temperature direct synthesis calorimetry. J Alloys Compd 350:205–212
Acknowledgements
This work was carried out as a sub task of ISS project of Fimecc Oy Elemet Program, supported financially by Tekes (Finnish Funding Agency for Technology and Innovation) and also Boliden Harjavalta, Boliden Kokkola, Norilsk Nickel Finland Oy as well as Outotec (Finland) Oy. The authors are also indebted to anonymous reviewers for providing insightful comments and giving two related papers as references.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Feng, D., Taskinen, P. Thermodynamic properties of silver–palladium alloys determined by a solid state electrochemical method. J Mater Sci 49, 5790–5798 (2014). https://doi.org/10.1007/s10853-014-8310-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-014-8310-4