Abstract
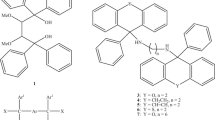
1,4-Bis(diphenylhydroxymethyl)benzene (H), a host compound possessing wheel-and-axle geometry, was found to possess host ability for dioxane (DIO), morpholine (MOR) and piperidine (PIP), forming inclusion compounds with each one with 1:2 host:guest ratios. This observation prompted an investigation of the host behaviour in mixtures of these guest compounds but where pyridine (PYR) was also considered (PYR was reported earlier to also form a 1:2 complex with H). In mixtures, H demonstrated significant affinities for, more especially, MOR and PIP, while DIO and PYR were usually disfavoured guest species. Single crystal X-ray diffraction experiments revealed that MOR (a favoured guest species) interacted by means of three hydrogen bonds with both adjacent guest and host molecules, plausibly explaining the host preference for this guest species; each of DIO, PYR and PIP were involved in only one interaction of this type with H. Total energy calculations revealed that the host-guest molecular pairs involving preferred MOR and PIP possessed significantly lower energies than those with disfavoured DIO and PYR. Thermal analyses demonstrated that the complex containing the least favoured guest compound, H‧2(DIO), possessed the lowest thermal stability of the four complexes, but these experiments did not clearly explain the affinity of H for MOR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many crystalline wheel-and-axle shaped compounds have demonstrated host ability. As early as 1968, Toda and Agaki reported on the inclusion properties of two such shaped compounds, namely 1,1,6,6-tetraphenylhexa-2,4-diyne-1,6-diol (1) and 1,1,4,4-tetraphenylbut-2-yne-1,4-diol (2) (Scheme 1) [1], which were capable of forming a number of inclusion compounds with a wide variety of organic molecules. The axles of these molecules are, more usually, long and linear, while the wheels are bulky and rigid and often comprise one or more aromatic moieties.
Numerous subsequent reports have been published with respect to host compounds with the wheel-and-axle design. As examples, Weber et al [2] reported on six novel host compounds based on two di(benzo[b]thien-2-yl)hydroxymethyl units that successfully enclathrated a number of different organic solvents, while Desiraju and co-workers [3] predicted accurately that 4-(triphenylmethyl)benzoic acid would form a wheel-and-axle host compound supramolecularly as a result of two molecules interacting, through their carboxylic acids, by means of hydrogen bonding. This supramolecule possessed the ability to form complexes, more especially with aromatic guest compounds such as xylene. Additionally, Nassimbeni and co-workers revealed the selectivity behaviour of 4,4’-bis(diphenylhydroxymethyl)diphenyl in mixed picolines [4].
In the present investigation, we observed that the wheel-and-axle compound 1,4-bis(diphenylhydroxymethyl)benzene (H, Scheme 1) has host ability for dioxane (DIO), morpholine (MOR) and piperidine (PIP), which prompted an investigation of the selectivity behaviour of H in mixtures of these guest compounds. Pyridine was also considered in these guest/guest competition experiments since it was previously reported to, similarly, form an inclusion compound with H [5]. From single crystal X-ray diffraction (SCXRD) experiments were identified influential intermolecular noncovalent interactions between host and guest, and/or guest and guest molecules, the cornerstone of self-assembly itself and supramolecular chemistry [6, 7], that explained the selectivity behaviour of this host compound in such mixtures. Toda and co-workers [8] were the first group to report on the inclusion behaviour of H with a great variety of organic compounds that possessed wide-ranging functional groups, while other research groups [9, 10] have focussed their attentions on amide and nicotine complexes with H. The behaviour of H in mixtures of these heterocyclic compounds (DIO, MOR, PYR, PIP) was never established in those works, and neither were provided any of the crystal structures of the complexes formed with them. In addition to SCXRD analyses on all successfully formed complexes in this work, thermal experiments were also carried out in order to determine and compare the relative thermal stabilities of each complex. We report on all of these findings here.
Experimental
Materials
All chemicals/solvents were purchased from Merck and were used without further manipulation.
Methods
1H-NMR spectroscopy
1H-NMR experiments were carried out in CDCl3 by means of a Bruker Ultrashield Plus 400 MHz spectrometer and the data were analysed by means of Topspin 3.2 software.
Single crystal diffraction (SCXRD) analyses
The crystal structure data of the three novel complexes produced in this work, namely H‧2(DIO), H‧2(MOR) and H‧2(PIP), were analysed at 296, 200 and 200 K, correspondingly, by employing a Bruker Kappa Apex II diffractometer with graphite-monochromated MoKα radiation (λ = 0.71073 Å). (H‧2(PYR) has been described on a previous occasion in an entirely different context [5].) APEXII was used for data collection while SAINT was employed for cell refinement and data reduction; SADABS was used for absorption corrections [11]. SHELXT-2018/2 [12] was used to solve the structures and refinements were carried out by means of least-squares procedures using SHELXL-2018/3 [13] together with SHELXLE [14] as a graphical interface. All non-hydrogen atoms were refined anisotropically. Carbon- and oxygen-bound hydrogen atoms were added in idealized geometrical positions in a riding model. The nitrogen-bound hydrogens were located on the difference Fourier maps and included in the refinement using riding models. Data were corrected for absorption effects using the numerical method implemented in SADABS [11]. The crystal structures of H‧2(DIO), H‧2(MOR) and H‧2(PIP) were deposited at the CCDC (Cambridge Crystallographic Data Centre), and their CCDC numbers are 2,271,343, 2,271,344 and 2,271,345, respectively (H‧2(PYR), CCDC 2,259,171).
Thermal analyses
Thermal experiments were conducted by employing a TA SDT Q600 module system. The complexes were first isolated from their solutions using vacuum filtration and, still under suction, these solids were washed with low boiling petroleum ether and then padded dry in folded filter paper. All data were analysed using either Pyris or TA Universal data analysis software. Samples were heated in ceramic pans with an empty pan serving as the reference; the heating rate was 10 °C‧min‒1 from 40 to 400 °C. The purge gas was high purity nitrogen.
Synthesis of 1,4-bis(diphenylhydroxymethyl)benzene (H)
This host compound was facilely prepared by considering a previous report [15].
Assessment of the host potential of H for DIO, MOR and PIP
In order to investigate whether H has host ability for DIO, MOR and PIP, this compound (0.1 g; 0.2 mmol) was dissolved, in glass vials, in each potential guest solvent (10 mmol) using mild heat to ensure complete dissolution where required. (Note that the host potential of H for PYR had been established previously [5], and a 1:2 host:guest (H:G) complex was isolated in that work.) The vials were kept open to the ambient conditions, and crystals formed in the solutions in this way. These were recovered by suction filtration (0.10 g of host compound produced 0.13 g of complex) and washed with low boiling petroleum ether, and then analysed by 1H-NMR spectroscopy. If complexation was successful, both host and guest resonance signals were observed on the spectrum, and the H:G ratio was obtained through a comparison of the integrals of relevant host and guest signals. These 1H-NMR spectra are provided in the Supplementary Information, Figures S1a–c.
Assessment of the selectivity behaviour of H in mixtures of DIO, MOR, PYR and PIP
The selectivity behaviour of host compound H was investigated in two separate sets of experiments, in equimolar mixtures involving all possible combinations of these guest solvents, and in binary mixtures where the molar amounts of two guest solvents present (Guest A and Guest B, GA and GB) were varied in sequence.
In the first of these, H (0.5‒0.7 g; 1‒2 mmol) was dissolved in equimolar binary, ternary and quaternary mixtures of these guests (10 mmol combined amount). The vials were closed and stored in a cold room (4 °C). After some time had passed, crystallization occurred, and these crystals were recovered as per the procedure in the single guest solvent experiments. Analysis was by means of 1H-NMR spectroscopy which allowed for the quantification of the guests present in the so-formed complexes as well as a calculation to obtain the overall H:G ratios.
In the second set of experiments, H (0.5‒0.7 g; 1‒2 mmol) was dissolved in solutions prepared by mixing two guest solvents (GA and GB) in ratios ranging from 20:80, 40:60 and 60:40 to 80:20. The vials were closed once more and stored in the cold room. The crystals that formed in this fashion were isolated as before and analysed by means of 1H-NMR spectroscopy in order to quantify the amounts of GA and GB in the so formed complexes. Selectivity profiles were then constructed by plotting the amount of GA (or GB) in the complex (ZA or ZB) against this amount in the original solution (XA or XB), as described by Pivovar et al. [16], according to the host selectivity equation KA:B = ZA/ZB x XB/XA (XA + XB = 1) (where K serves as a measure of the extent of the host selectivity). Also inserted into these profiles are straight lines that represent a host compound that is unselective (and K = 1) so that this scenario may readily be compared with the experimental data points.
Software
SCXRD data were analysed by means of program Mercury [17]. In so doing, the unit cells, space groups, bond lengths and angles, and noncovalent intra- and intermolecular interactions could all be identified. Furthermore, by deleting the guest molecules from the packing diagrams, the type of guest accommodation could be ascertained (whether in continuous channels or in discrete cages): the spaces that remained after these deletions that could accommodate a probe with a 1.2 Å radius could thus be visualized and are the voids in which the guests were housed. Program Crystal Explorer 17.5 was employed for the energy calculations reported herein [18].
Results and discussion
Assessment of the host potential of H for DIO, MOR and PIP
When H was independently crystallized from DIO, MOR and PIP, complexation occurred in each instance, and the results are summarised in Table 1.
The H:G ratios of all complexes formed in this way were consistently 1:2 (Table 1).
Assessment of the selectivity behaviour of H in mixtures of DIO, MOR, PYR and PIP
Table 2 contains the selectivity data that were obtained when H was crystallised from equimolar mixtures of DIO, MOR, PYR and PIP. The G:G and overall H:G ratios were obtained from 1H-NMR spectroscopy experiments. The favoured guests are in red bold font face, and all experiments were carried out in duplicate, and hence the percentage standard deviations (% e.s.d.s) are also provided in this table.
From the equimolar binary experiments, MOR was a particularly preferred guest species, more especially when PIP was absent: DIO/MOR and MOR/PYR solutions furnished complexes with 92.7 and 95.3% MOR, respectively. In the absence of MOR, however, significant H selectivities were still observed: DIO/PYR, DIO/PIP and PYR/PIP solutions afforded inclusion compounds with 88.8, 91.7% and 95.5% PYR, PIP and PIP, correspondingly. Only in the case where both MOR and PIP were present was there noted some competition: MOR remained favoured, however, but the crystals now only contained 72.7% MOR. Interestingly, the ternary experiments containing DIO/MOR/PYR and MOR/PYR/PIP produced complexes with enhanced quantities of MOR and PIP (90.0 and 88.1%). The latter result was not an expected one since MOR was always selected for in every other experiment where it was present, but here, where PIP was now significantly favoured (88.1%) despite the presence of MOR. (This experiment was conducted in duplicate, and comparable results were obtained in each instance, as indicated by the low %e.s.d. values, Table 2.) The reason for this observation is not currently clear. In the remaining experiments, both ternary and quaternary in nature, the selectivity of H decreased significantly (54.1‒68.9%), but MOR was consistently favoured in experiments involving it. Note that in all of these experiments, DIO remained disfavoured in every instance. However, the DIO/PYR/PIP experiment resulted in a mixed complex with as much as 43.4% DIO (though PIP was preferred, as expected, 54.1%). This was, once more, not an expected result and the reason for this observation is also unclear.
The selectivity profiles that were constructed from the binary GA:GB mixtures where the molar ratios of the two guests were sequentially varied are provided in Fig. 1a‒f.
As expected, given the equimolar solution data in Table 2, experiments in MOR/DIO solutions (Fig. 1a) revealed that MOR was consistently preferred across the concentration range. Even at low concentrations of MOR in the solution, significant quantities of this guest were extracted by H: as an example, when only 40% MOR was present in the mixture, the complex already contained 96% MOR. Similar significant selectivities were noted in PIP/DIO (Fig. 1c) and MOR/PYR (Fig. 1d) solutions: in the former, 40% PIP in the solution furnished crystals with as much as 92% PIP, while the crystals isolated in the latter from a solution comprising 20% MOR were enriched in MOR (95%). In the case of experiments in DIO/PYR (Fig. 1b) and PYR/PIP (Fig. 1e), the selectivity of H was usually for the guest that was present in the greater concentration. Finally, and unsurprisingly, experiments in MOR/PIP solutions demonstrated that, despite MOR being favoured across the concentration range, this selectivity was not extreme, and all data points are close to the line represented by K = 1 for an unselective host compound.
These data clearly confirm that MOR is a guest solvent that is extremely preferred by H, and also PIP to some extent, while both DIO and PYR are guest solvents less favoured by this host compound. Our research group was intrigued to identify the reasons pertaining to these selectivity observations, and so SCXRD experiments were carried out on each single solvent complex formed in this work.
Single crystal diffraction (SCXRD) analysis
The relevant crystallographic data for the H‧2(DIO), H‧2(MOR) and H‧2(PIP) complexes are summarized in Table 3. All of these inclusion compounds crystallized in the monoclinic crystal system and space group P21/c (H‧2(PYR), however, crystallized in the triclinic crystal system and space group P‾1 [5]) (Table 3). Two disorder components of each of DIO, MOR and PIP were noted in their complexes (while PYR did not experience disorder in its inclusion compound with H [5]). Figure 2a‒d are illustrations of the unit cells and host-guest packing (left) and the voids remaining after guest deletion from the packing calculations (yellow, right). (Guests are in spacefill and hosts in stick representation.)
From the void diagrams, all guest molecules were accommodated in infinite unidirectional channels, along the a-axis in the case of H‧2(PYR) (Fig. 2d) [5] and the c-axis in the remaining three complexes (Fig. 2a‒c).
The geometry of the host molecule in each complex was always centrosymmetric around an inversion point positioned in the centre of the central aromatic ring.
In H‧2(DIO), no significant (host)π‧‧‧π(host) interactions could be identified. However, the DIO guest species was retained in the crystal of the complex by means of (host) O‒H‧‧‧O (guest) classical hydrogen bonding interactions involving both components of guest disorder (interestingly, only one of the two oxygen atoms of each guest molecule experienced this interaction type). These measured 2.769 (3) and 2.82 (2) Å (D‧‧‧A) (1.99 and 2.03 Å (H‧‧‧A)) and the respective angles (O‒H‧‧‧O) were 158 and 160°. Figure 3 is a stereodiagram depicting these interactions (here, one disorder component was deleted for clarity).
Additionally, both disorder components of DIO experienced (guest) C‒H‧‧‧π(host) interactions with H (H‧‧‧π 2.66‒2.98 Å, C‒H‧‧‧π 125‒147°), two contacts of this type being identified for one of the disorder components (Fig. 4) and one for the other.
Once more, there were no significant (host)π‧‧‧π(host) interactions present in H‧2(MOR). However, both disorder components of MOR experienced (host) O‒H‧‧‧N(guest) H-bonding interactions, and these measured 2.825(3) and 2.822(10) Å (O‧‧‧N) with angles 169 and 164° (H‧‧‧N distances were both 2.00 Å); additionally, (guest)N‒H‧‧‧O(guest) interactions were also identified, resulting in endless ribbons of H-bonded guest molecules in this complex. Here, N‧‧‧O distances were 3.191(3) and 3.268(9) Å (H‧‧‧O 2.35 and 2.40 Å) (155, 163°). These are illustrated in Fig. 5 (a stereoview, top, and the endless ribbons of guest molecules, bottom) for the one disorder guest component only.
A stereoview demonstrating the host‧‧‧guest and guest‧‧‧guest H-bonding interactions (green) in H‧2(MOR) (top) and a depiction of the endless ribbons of H-bonded guest‧‧‧guest molecules (bottom) that resulted (the H-bonded arrangement shown in the top stereoview is repeated by inversion at the bottom left of that figure but is not shown here for simplicity). (Color figure online)
Furthermore, and as was the case in the DIO-containing inclusion compound, MOR also experienced (guest)C‒H‧‧‧π(host) stabilizing contacts with the aromatic moieties of the host compound, and both components of the disordered guest were involved in two such interactions; H‧‧‧π distances ranged between 2.68 and 2.92 Å (144‒147°). Figure 6 is an illustration and was prepared by considering, once more, only one of the guest disorder components for clarity.
In a similar fashion, no significant (host)π‧‧‧π(host) contacts were observed in the complex containing PIP but, once more, PIP was involved in (host)O‒H‧‧‧N(guest) interactions with the host molecule; these were also experienced by both disorder components, and O‧‧‧N distances were 2.810(3) and 2.626(16) Å (H‧‧‧N 1.97 and 1.81 Å) (174, 163°). Figure 7 is a stereoview to illustrate these interactions but where one of the two guest disorder components has been deleted.
Additionally, each H molecule interacted with two PIP molecules by means of (guest) C‒H‧‧‧π(host) interactions, and only one of the two components of disorder was involved in this fashion. Measurements (H‧‧‧π) were 2.74 Å and 149° (Fig. 8).
It has been reported that PYR, in its complex with H, is also H-bonded to the host molecule (O−H‧‧‧N 2.8195(16) Å; H‧‧‧N 2.01 Å, 168°) [5]. This guest species, furthermore, interacted with a neighbouring guest molecule through π‧‧‧π contacts (3.779 (1) Å), while C‒H‧‧‧π interactions were also identified in the complex.
Subsequently, Crystal Explorer 17.5 software18 was employed to calculate the energies experienced by each host-guest molecular pair. Each disorder guest component in the case of DIO, MOR and PIP was considered in turn. Using this program, the host and guest molecules were selected after having completed all the molecular fragments, and then the total energy calculated for the pair at the HF/3-21G level (the total energy is comprised of electrostatic, polarisation, dispersion, and exchange–repulsion contributions). Table 4 summarises the energy data that was so obtained.
These energy data (Table 4) clearly suggest that the lowest energy host-guest molecular pairs (and hence the more stable) are those having the preferred MOR and PIP guest species; total energies were much lower (ranging between ‒59.4 and ‒66.6 kJ‧mol‒1) than in the complexes with disfavoured guests, DIO and PYR. These latter two molecular pairs were significantly less stable since their energies were higher, ranging between ‒40.3 and ‒48.0 kJ‧mol‒1.
In summary, the affinity of H for MOR is attributed to the fact that this guest molecule experiences three stabilizing hydrogen bonds, two N−H‧‧‧O H-bonds with neighbouring guest molecules and one O−H‧‧‧N H-bond with the host molecule; all other guest molecules in this work are involved in only one contact of this type with H. Furthermore, host-guest molecular pairs involving the preferred guests MOR and PIP experienced increased stabilities compared with those having DIO and PYR, as indicated by the energy calculations.
Thermal analyses
The relative thermal stabilities of each of the four complexes was ascertained through thermoanalytical experiments, and the resultant thermal traces (overlaid DSC, TG and DTG) are provided in Figure S2a–c of the Supplementary Information, while Table 5 summarises the relevant data that were obtained from these.
The measured mass losses were in close agreement with those expected for each of the 1:2 complexes produced in this work (Table 5). From the guest/guest competition experiments (Table 2), it was observed that the host selectivity behaviour was in the order MOR > PIP > PYR > DIO (Table 2). The Ton values provided in Table 5, the temperature at which the guest release process commenced, and which serves as a measure of the relative thermal stabilities of these complexes, demonstrates that, indeed, the DIO-containing complex with the least favoured guest solvent possessed the lowest thermal stability of the four, and Ton was only 47.2 °C. However, H‧2(PYR) (containing the second least preferred guest), surprisingly, displayed a significantly enhanced stability (Ton 59.7 °C) compared with the complexes of each of the remaining guests, even those with the more preferred MOR (Ton 51.8 °C) and PIP (Ton 53.4 °C). These stability data, therefore, do not concur wholly with the host selectivity observations made in the competition experiments (Table 2).
Conclusions
1,4-Bis(diphenylhydroxymethyl)benzene (H) was a successful host compound when presented with each of DIO, MOR and PIP, forming 1:2 H:G inclusion complexes with each one (PYR was also included with the same ratio, as previously reported). In various equimolar and non-equimolar guest/guest mixtures, H demonstrated a significant affinity for MOR, while DIO was never preferred in any of these crystallization experiments. The host selectivity was determined to thus be in the order MOR > PIP > PYR > DIO. SCXRD experiments showed that the preferred guest species, MOR, formed three hydrogen bonds, two with adjacent guest molecules and one with the host compound, while DIO, PIP and PYR experienced only one interaction of this type with H, thus explaining the preference of H for MOR. Present in all four complexes were (guest)C‒H‧‧‧π(host) interactions. Also, the host-guest molecular pairs in the MOR- and PIP-containing complexes with preferred guests were more stable than those with disfavoured DIO and PYR, as indicated by total energy considerations. Thermal analysis revealed that the complex with the least preferred guest compound (DIO) possessed the lowest thermal stability of the four, but could not explain the affinity of H for MOR.
References
Toda, F., Agaki, K.: Molecular complexes of acetylene alcohols with n- and ê“-donors. Tetrahedron Lett. 9, 3695–3698 (1968)
Katzsch, F., Gruber, T., Weber, E.: Structural studies on inclusion compounds and solvent sorption behavior of gradually elongated wheel-and-axle-type diol hosts featuring lateral benzo[b]thiophene units. J. Mol. Struct. 1114, 48–48 (2016)
Jetti, R.K.R., Kuduva, S.S., Reddy, D.S., Xue, F., Mak, T.C.W., Nangia, A., Desiraju, G.R.: 4-(Triphenylmethyl)benzoic acid: A supramolecular wheel-and-axle host compound. Tetrahedron Lett. 39, 913–916 (1998)
Nassimbeni, L.R., Su, H., Weber, E., Skrobidis, K.: Structures of 4,4’-bis(diphenylhydroxymethyl)diphenyl with picolines: Selectivity and phase transformation. Cryst. Growth Des. 4, 85–88 (2004)
Barton, B., Vorgers, J., Hosten, E.C.: The behaviour of the wheel-and-axle host compound 1,4-bis(diphenylhydroxymethyl)benzene in mixed pyridyl guest solvents. Cryst. Growth Des. (2023). https://doi.org/10.1021/acs.cgd.3c00548
Atwood, J.L., Steed, J.W.: Encyclopedia of supramolecular chemistry, vol. 1. CRC Press, New York (2004)
Steed, J.W., Atwood, J.L.: Supramolecular Chemistry. Wiley, USA (2009)
Toda, F., Kai, A., Toyotaka, R., Yip, W.-H., Mak, T.C.W.: New hosts bearing two triphenylcarbinol groups and crystal structures of their acetone inclusion compounds. Chem. Lett. 18(11), 1921–1924 (1989)
Jacobs, A., Nassimbeni, L.R., Silwana, N., Báthori, N.B., Weber, E.: Inclusion of 1,4-bis(diphenylhydroxymethyl)benzene with amides: Structure and selectivity. CrystEngComm. 13, 7014–7018 (2011)
Capucci, D., Balestri, D., Mazzeo, P.P., Pelagatti, P., Rubini, K., Bacchi, A.: Liquid nicotine tamed in solid forms by cocrystallization. Cryst. Growth Des. 17, 4958–4964 (2017)
Bruker, A.: APEX2, SADABS and SAINT. Bruker A.X.S., Madison (2010)
Sheldrick, G.M.: SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. A71, 3–8 (2015)
Sheldrick, G.M.: Crystal structure refinement with SHELXL. Acta Crystallogr. C71, 3–8 (2015)
Hübschle, C.B., Sheldrick, G.M., Dittrich, B.: ShelXle: A qt graphical user interface for SHELXL. J. Appl. Crystallogr. 44, 1281–1284 (2011)
Weber, E., Skobridis, K., Wierig, A., Nassimbeni, L.R., Johnson, L.: J. Chem. Soc. Perkin Trans. 2, 2123–2130 (1992)
Pivovar, A.M., Holma, K.T., Ward, M.D.: Shape-selective separation of molecular isomers with tunable hydrogen-bonded host frameworks. Chem. Mater. 13, 3018–3031 (2001)
Macrae, C.F., Sovago, I., Cottrell, S.J., Galek, P.T.A., McCabe, P., Pidcock, E., Platings, M., Shields, G.P., Stevens, J.S., Towler, M., Wood, P.A.: Mercury 4.0: From visualization to analysis. Design and prediction. J. Appl. Crystallogr. 53, 226–235 (2020)
Wolff, S.K., Grimwood, D.J., McKinnon, J.J., Jayatilaka, D., Spackman, M.A.: Crystalexplorer 17.5. University of Western Australia, Perth (2007)
Acknowledgements
Financial support is acknowledged from the Nelson Mandela University and the National Research Foundation (NRF). MRC thanks the University of Cape Town for access to research facilities.
Funding
Open access funding provided by Nelson Mandela University.
Author information
Authors and Affiliations
Contributions
BB Conceptualization; Funding acquisition; Methodology; Project administration; Resources; Supervision; Visualization; Writing—original draft. MRC Resources; Visualization; Formal analysis. JV Investigation; Methodology; Validation. ECH Data curation; Formal analysis.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10847_2023_1210_MOESM1_ESM.docx
The crystal structures of H‧2(DIO), H‧2(MOR) and H‧2(PIP) were deposited at the Cambridge Crystallographic Data Centre; these data are available free of charge from www.ccdc.cam.ac.uk/data_request/cif; the CCDC numbers are 2,271,343, 2,271,344 and 2,271,345, respectively (H ‧2(PYR), CCDC 2,259,171). In the Supporting Information were deposited the 1H-NMR spectra of the three novel single-solvent complexes as well as their thermograms. Supplementary material 1 (DOCX 235 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Barton, B., Caira, M.R., Vorgers, J. et al. Investigation of the hydrogen bonded host‧‧‧guest and guest‧‧‧guest interactions present in complexes of a polyaromatic wheel-and-axle host compound with dioxane, morpholine, piperidine and pyridine. J Incl Phenom Macrocycl Chem 104, 15–24 (2024). https://doi.org/10.1007/s10847-023-01210-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-023-01210-4