Abstract
The high Andean mountains are ecosystems subject to high human pressure activities, resulting in disturbed areas increasingly dominating the landscapes. However, there needs to have more knowledge about the contributions of different vegetation coverages and species to global diversity at the local level. For three consecutive years, we studied a guild of fruit-feeding butterflies in four different land covers (cloud forests, paramo, mixed anthropogenic habitats, and cattle pastures) in the northern Andes in Colombia, analyzing the diversity and structure of the butterfly assemblage in the region. The assembly showed significant differences between land covers. The mixed habitat was the most diverse in order q1 (exponential of Shannon entropy) and q2 (inverse of Gini-Simpson dominance index) diversity, and the cloud forest contained the higher abundance and species richness. Abundance decreased from forest to pasture, significantly reducing diversity in pastures, with cloud forest and paramo containing the key endemic species. These results indicate the value of landscape diversity in providing resources and conditions required for the diversity conservation of high Andean butterflies.
Implications for insect conservation
This study highlights the significance of the heterogeneity of natural landscape components in maintaining and preserving the diversity of butterfly assemblages in high Andean environments. Current conservation plans often tend to focus on paramo habitats, our findings highlight the crucial role of including the surrounding cloud forest and associated secondary forest in the design of effective conservation strategies. The analysis revealed a high complementarity between paramo and cloud forest assemblages, with each habitat type contributing significantly to the regional species pool. This underscores the interconnectedness and interdependence of these habitats, indicating that a comprehensive approach that considers both paramo and cloud forest ecosystems is essential for conserving the full diversity of butterfly species in the region. Moreover, our results demonstrate that cattle pastures exhibit extremely low butterfly species richness and possess a community structure that is distinctly different from native habitats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The tropical Andes, one of the most diverse regions in the world, face significant environmental challenges. For instance, over 2000 bird species, ca. 22% of all known species, inhabit the region, and almost 600 (28%) of them occur nowhere else on Earth (Herzog and Kattan 2011). Of South America’s five hotspots, the tropical Andes is the most diverse and with more endemic species: fish (74%), amphibians (71.4%), vascular plants (50%), reptiles (40%), and mammals (13.3%) (CEPF 2021). While the exact number of butterfly species is unknown, recent estimates indicate that there are over 4000 species in the tropical Andes, many of which are confined to the high mountain habitats (Pyrcz et al. 2014, 2016). The high levels of endemism and diversity in the Andean region make it highly vulnerable to even minor environmental changes, which can profoundly impact local assemblages. Unfortunately, the region has been severely affected by the global biodiversity crisis concerning the Anthropocene. The area has a high human population density and has experienced the continuous conversion of natural habitats for economic activities such as agriculture, livestock farming, mining, and urbanization (Etter and van Wyngaarden 2000; Bax et al. 2019), resulting in a considerable transformation of the original vegetation into a mosaic of crops and pastures interspersed with fragments of native habitat (Otero and Onaindia 2009). The extent of this conversion is alarming, with less than 50% of the original vegetation remaining (Armenteras et al. 2003; Rangel-Ch 2005), with the tropical high Andean original vegetation mainly transformed into cattle pastures and potato crops. Furthermore, tropical mountain insect species are threatened not only by human activities but also by the impacts of climate change in recent years (Peyre et al. 2020). Climate projections suggest that variations in temperature and precipitation will significantly impact the potential distribution areas of different species of tropical high mountain butterflies, with scenarios that estimate a significant contraction (between 30 and 56%) of suitable climatic areas (Moguel-Cárdenas et al. 2024). The combined effect of these factors makes the tropical Andes one of the most endangered regions globally.
Naturally, the tropical Andes are divided into altitudinal belts that form different ecosystems from the lowland rain forest to the high mountain tundra ecosystem, named paramo, in the northern section of the Andes (páramo in Spanish) (Cuatrecasas 1958; van der Hammen 1974). These belts are conformed by characteristic ecological assemblages, resulting in a high beta diversity along the altitudinal gradient. Some groups, such as butterflies and moths, respond more precisely to these changes, showing significant differences in the species assemblage along the altitudinal gradient (Brehm et al. 2003; Pyrcz et al. 2009; Marín et al. 2015). For these groups, climate change represents one of the main threats, with temperatures along tropical mountains increasing 0.017 °C per year, and ecosystems are already shifting upslope (Feeley et al. 2013; Harvey et al. 2023), being documented for some species of carabid beetles from high Andean mountains in Ecuador an upward shift of their upper limit of approximately 400 m in 28 years (Moret et al. 2016).
The fruit-feeding butterflies have been studied extensively in tropical regions to understand the environmental and ecological changes at different landscape scales, being used successfully in studies of land-use conversion (Barlow et al. 2007, 2008; Casas-Pinilla et al. 2022), landscape fragmentation (Uehara-Prado et al. 2007; Uehara-Prado and Freitas 2009; Ribeiro et al. 2012; Melo et al. 2019), perturbation gradients (Ribeiro and Freitas 2012; Iserhard et al. 2019; Spaniol et al. 2019; Bellaver et al. 2022), and transitions between natural and anthropogenic habitats (Lourenço et al. 2019). The use of fruit-feeding butterflies in ecological studies is a result of their diversity, with more than 1600 species in the Neotropical region, with above 40% of the total species diversity of Nymphalidae in the lowland forest (Santos et al. 2011) and above 70% of the diversity in the montane forest (Marín et al. 2014). Thus, these insects are highly sensitive to environmental alterations and have been widely used as biological indicators (Blair and Launer 1997; Matsumoto 2015; Rödder et al. 2021; Mtui et al. 2022). However, studies with fruit-feeding butterflies are mostly restricted to lowland habitats. Low performance of fruit-baited traps is frequently registered in montane environments (including the Andean region), compared to those in lowland forests (see Caldas and Robbins 2003); this is the result of the poor understanding of the natural history of the species in Andean high altitude environments (Shapiro 1992; Álvarez et al. 2021).
The present study describes the alpha and beta diversity of fruit-feeding butterfly assemblages from the high-altitude Andean region under different anthropogenic intervention grades in four habitats with different land covers (cloud forest, paramo, mixed areas, and cattle pastures) and distinct degrees of disturbance within the influence area of the Páramo de Belmira (Santa Inés) in the highlands of the northern Colombian Andes. Thus, we expect higher richness and low dominance (alpha diversity) between paramo and forest habitats with a reduction of diversity (lower richness and increasing dominance) in areas with high anthropogenic intervention. Moreover, the composition of fruit-feeding butterflies (beta diversity) will be distinct between paramo and the other habitats given the intrinsic characteristics of this unique vegetal formation in Andes.
Materials and methods
Study site and fieldwork
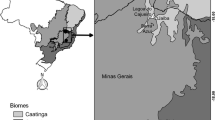
The study was carried out in the north of the Cordillera Central of the Colombian Andes in the Páramo de Belmira protection area (6°35’–6°51’ N and 75°47’–75°38’ W) (see: Pyrcz et al. 2016). The regional landscape is dominated by four different land covers (Fig. 1); (1) Cloud forest (hereafter “forest”): on the Andean ridge flanks between 2,000 and 3,200 m a.s.l., this vegetation is characterized by middle-size trees (around 12 m high) with abundant epiphytes hanging from tree trunks and branches. The cloud forest is frequently embedded in fog, the mean temperature is below 15 °C, air humidity is high, and solar radiation is irregular and predominantly low (Tobón 2021); (2) Paramo: a high-altitude ecosystem of Central and northern South America, located at the cloud forest’s upper limit above 3,000 m a.s.l. The paramo is open area vegetation dominated by native grasses, large rosette plants, and shrubs (Cuatrecasas 1958; van der Hammen 1974); (3) Cattle pastures (hereafter “pasture”): an artificial, highly disturbed habitat dominated by non-native grasses (such as Pennisetum clandestinum Hochst. & Chiov.) with few scattered trees, designed for extensive cattle ranching; and (4) Mixed habitat a mosaic of cloud forest, paramo, and abandoned cattle pastures in various successional stages without an apparent prevalence of a single vegetation type.
Sampling was conducted monthly from June 2011 to April 2014 in 12 localities (three replicates for each of the four land covers) between 2,600 and 3,300 m a.s.l. A sampling unit composed of four standard Van Someren-Rydon (VSR) traps was installed in each locality. Traps were placed about 1.5 m above ground and separated 100 m from each other, baited alternately with fermented bananas (mixture of mature banana hand mashed with rum and sugar) and rotten fish (trout) (for more details, see Freitas et al. 2014 and Álvarez et al. 2021). Traps were revised three times over four days.
Data analyses
The diversity and composition of butterfly assemblages in the different land covers were compared using several approaches. First, diversity profiles based on Hill numbers through the q statistic were calculated (Hill 1973; Jost 2006), species richness is q = 0, the diversity of order q = 1 representing the exponential of Shannon entropy concerning equitability, and q = 2 representing the inverse of the Gini–Simpson dominance index. Hill numbers offer advantages over traditional diversity indices, with the replication principle or doubling property that is of intuitive interpretation of the same way that species richness for the different diversity profiles. Furthermore, they are all expressed in units of effective numbers of species, and the order of diversity indicates its sensitivity to common and rare species, the diversity of all species (q0), of ‘‘typical’’ species (q1), and of dominant species (q2) (Chao et al. 2014). Richness among land covers was compared by rarefaction based on Hurlbert (1971) using rarefy function in vegan 2.5-6 Oksanen et al. (2019). Finally, the sample coverage (Chao and Shen 2003) was estimated for each habitat in the package iNEXT 2.0.20 (Chao et al. 2014; Hsieh et al. 2020) as an indicator of the proportion of the total number of individuals that belong to the species represented in the sample, being a measure of sample completeness (Chao and Jost 2012).
An analysis of abundance distributions was performed for each assemblage with a rank-abundance curve (Whittaker 1965; Wilson 1991), displaying logarithmic species abundances against species rank order. The selection of the fitting model to the rank-abundance curve (Log-Normal, Geometric, Broken Stick, Zipf, and Zipf-Mandelbrot) was made with Akaike Information Criterion, using rarfit function in vegan 2.5-6 package (Oksanen et al. 2019).
To test differences among assemblages we used the permutational multivariate analysis of variance (PERMANOVA) based on Anderson’s algorithm (2001) and the Bray-Curtis dissimilarity measure. Nonmetric Multidimensional Scaling (nMDS) of two dimensions was used to visualize assemblage composition in relation to habitat. A SIMPER (similarity percentage) (Clarke 1993) procedure was applied to know the contribution of a single species to the overall differentiation between habitats. One million permutations based on the decomposition of the Bray-Curtis dissimilarity index were employed to evaluate the differences among habitats. Pairwise comparisons were made to find the contribution of each species to the Bray-Curtis dissimilarity. The packages MASS 7.3–51.5 (Venables and Ripley 2002) and Vegan 2.5-6 (Oksanen et al. 2019) in R 3.6.3 (R Core Team 2020) were used for PERMANOVA, nMDS, and SIMPER analyses. The SIMPER analysis was complemented with an Indicator Species Analysis (IndVal), an alternative to SIMPER for identifying species indicative of given groups but including a plot per species. This approach combines the relative species abundance with its frequency of occurrence in the various groups of sites, calculated as a percentage value (0-100), by the formula IndValij = Aij x Bij x 100, where for each species i in each site group j, it was computed the product of Aij, which is the mean abundance of species i in the sites of group j compared to all groups in the study, by Bij, which is the relative frequency of occurrence of species i in the sites of group j (Dufrêne and Legendre 1997). The statistical significance (p values), Bonferroni corrected, was estimated by 9999 random reassignments (permutations) of sites across groups. The analysis was performed in PAST v 4.07 (Hammer et al. 2001).
Results
Overall, we recorded 2,939 individuals belonging to 57 species of fruit-feeding butterflies from two subfamilies of Nymphalidae (Satyrinae and Biblidinae) (Table 1, Supplementary Table 1); no Charaxinae and Nymphalinae were found throughout the study. The sample coverage was upper of 91% in all land covers; in which the forest cover had the highest values of richness with 47 species and about half of the registered individuals (n = 1472), followed by mixed habitats and paramo; while the pasture cover had the lowest richness and abundance with 21 species and 151 individuals (Table 2). The rarefaction analysis (Fig. 2A) showed a similar pattern, with pasture indicating the lowest richness. The results of the diversity profile indicate that the high diversity was found in the mixed habitat (high evenness and low dominance), forest and paramo had closed values of diversity following the mixed habitat, and pasture presented a high dominance and low evenness. The most abundant species in each land cover were Satyrinae, with Pedaliodes obstructa Pyrcz & Viloria, 1999 in forest and mixed habitat, Altopedaliodes belmira Pyrcz, and Rodriguez, 2004 in the paramo, and Panyapedaliodes drymaea (Hewitson, 1858) in the pasture cover. Rank-abundance distributions analysis indicates different models for each land cover; the forest and mixed habitat both fit the Log-Normal model, paramo fits the Geometric and Zipf-Mandelbrot models, and the pasture fits the Zipf-Mandelbrot model (Fig. 2B).
The PERMANOVA showed significant differences among the assemblages, corroborating the distinct clusters of composition of fruit-feeding butterflies in each habitat as showed in the ordination space of the nMDS analysis (Fig. 3). Moreover, the SIMPER indicates high dissimilarities between habitats, with a minimum between forest and mixed habitat of 0.587 and a maximum between forest and pasture of 0.940 (Table 3). The distinction among them is determined by eighteen species that explain more than 70% of the dissimilarity. Likewise, the IndVal analyses show ten indicators species that emerged with statistically significant values upper to 50% in the habitats of forest, paramo, and pasture and no indicator species for the mixed habitat (Fig. 4): Forsterinaria rustica (A. Butler, 1868), Pedaliodes baccara Thieme, 1905, P. obstructa, and P. rodriguezi Pyrcz & Andrade, 2013 are recovered as indicators of forest, A. belmira, Junea dorate (Hewitson, 1858), L. circe C. Felder & R. Felder, 1859, Pedaliodes nutabe Pyrcz & Alvarez, 2016, and P. polusca (Hewitson, 1862) are indicators of paramo, and P. drymaea is recovered as an indicator of pasture.
The comparison between forest and paramo suggests that these two natural habitats are complementary, with a high dissimilarity (upper 70%) between them. The paired SIMPER procedure highlighted the importance of relatively few species in all habitats (less than 20% of the recorded ones with baited taps). Two species, P. obstructa, and P. baccara contributed to the main differentiation between the forest and other land covers. Pronophila epidipnis was the most important species to differentiate mixed habitats from paramo and pasture, while A. belmira, P. drymaea, and P. polusca were determinants differentiating between paramo and pasture.
Discussion
Our results agree, partially, with the initial hypotheses indicating that the high Andean fruit-feeding butterfly assemblages respond to the differences in habitat, showing a higher richness and abundance in the forest, but mixed habitats had more diversity (high evenness and low dominance); pasture had lower richness, abundance and diversity values. Concerning beta diversity, there is a significant segregation in species composition between paramo and, mainly, forest habitats, but all studied habitats have a peculiar subset of species composition.
The structure of fruit-feeding butterfly assemblages exhibits a distinct pattern in terms of alpha diversity, as revealed by analyses of range abundance curves and diversity profiles (Fig. 2B). Although the use of species–abundance distribution models has been widely debated (Williamson and Gaston 2005; Ferreira and Petrere 2008), these are helpful predictive tools for the structure and evenness of communities (McGill et al. 2007), being able to help improve predictions and responses of the species to climate change and to understand the success of different conservation strategies (Mair et al. 2014). The species-abundance model distribution in forest and mixed habitats correspond to communities where a hierarchical niche subdivision occurs and may indicate that a small fraction of species uses a large portion of the available resources. In this way, the log-normal distribution may indicate a complete portrait of these two habitat types, in which the addition of more individuals as the sample increases reveals this pattern distribution. So, as the abundance of fruit-feeding butterflies increased, fewer species became rare, showing a suitable distribution of butterflies in these habitats, probably indicating equality in the use of resources (Iserhard et al. 2017). For paramo, the butterfly assemblage fits equally well at two different models, Geometric Series and Zipf-Manldelbrot. In the Geometric Series or Niche Preemption model, the abundance of a species in the community depends on the niche occupied by its predecessor, where each species takes a constant fraction of the remaining resources, which implies an assembly with high dominance (McGill et al. 2007).
Pastures fit at two similar models, Zipf and Zipf-Manldelbro, such as the paramo; this result suggests an assemblage with a high dominance with fewer species with great abundance. Although paramo and pasture are two distinct assemblages, we suggest that both assemblages have highly dominant species that would result from environment filters that constrain the ingress of new species to the assemblage. It is different filters, with a natural ecological filter in paramo that can be corroborated with the presence of several diversified species groups and species mainly distributed in this habitat, such as L. circe, J. doreate, and endemic species such as A. belmira, P. nutabe, and Panyapedaliodes rojasi Pyrcz & Alvarez, 2016. On the contrary, an artificial filter acts on cattle pastures, where the anthropogenic activity favors a few species of the regional pool with the capacity to survive in this habitat. Thus, the high disproportion of pasture with other habitats may result from the presence of only a few non-resident species with high abundance. These differences are possible because of the high disturbance level of pasture, where native plant communities are replaced by non-native plants, resulting in a significant bottom-up reduction of energy available for local food webs affecting both specialist and generalist butterflies (Burghardt et al. 2010).
The beta diversity analyses indicate that several species were characteristically associated with each habitat, indicating the higher faunal differentiation among habitat types. The formation of distinct species assemblages among the studied land covers is corroborated by the diversity distribution and variation in the studied area through the ordination analysis. P. baccara and P. rodriguezi were characteristics of the forest habitat, A. belmira, J. doreate, L. circe, and P. nutabe were characteristics of the paramo, and P. drymaea is tolerant to highly perturbed pasture habitat. Thus, three of the studied habitats, the forest, mixed habitat, and paramo, contribute differentially to local butterfly diversity, whose differences in the perturbation regimes and land cover types at the landscape level could partially explain this pattern. Accordingly, the mixed habitat with a middle perturbation has no indicator species, showing a species mix between forest and paramo assemblages. The high specificity for some habitats would indicate that local habitat conditions filter those species from the regional pool whose requirements are best met at the respective sites (Kraft et al. 2007; Rabl et al. 2020). Mainly, Andean mountain tops act as a filter for species from lower elevations with significant isolation, showing a high species turnover among mountain massifs (Álvarez-Hincapié et al. 2017; Mahecha et al. 2019), in which endemic species develop and survive with a significant correlation between endemism and topographic isolation exists (Adams 1985). However, it is necessary to consider that species’ habitats go beyond vegetation categories and that there is a need to fully understand the relationship between valid habitat species and identifiable units in the landscape that can be used for recording and managing biodiversity (Dover et al. 2010; Dennis et al. 2014).
The current study suggest that fruit-feeding butterflies in the Andean mountains exhibit distinct patterns of differentiation at the local scale, particularly between the forest and paramo ecosystems. This indicates a significant divergence in the composition of butterfly assemblages, highlighting the crucial role of environmental heterogeneity in ecological processes. Furthermore, it emphasizes the importance of considering different land-use systems within a diverse landscape (Bellaver et al. 2022; Lorandi et al. 2023). These findings are in line with previous studies (Kraft et al. 2007; Rabl et al. 2020) that have underscored the significance of habitat specificity in influencing species distributions. They highlight the need to conserve these mountain habitats to safeguard the rich biodiversity and unique evolutionary processes that have given rise to the remarkable endemism’s found in the Andean region. The results of our study emphasize the intricate interplay between microhabitat conditions and landscape dynamics in shaping butterfly diversity, suggesting that a comprehensive approach is required to manage these ecosystems effectively. Further research is needed to develop robust management plans that ensure the long-term viability of these diverse habitats and the preservation of their biodiversity.
In conclusion, our study shows the fundamental role of native habitats in promoting the diversity of Andean butterflies, including specific resource availability and optimal environmental characteristics for this group of insects. Understanding these relationships is essential for developing effective strategies that take into account the specific requirements of different species and the ecological integrity of their habitats. This may increase the survivorship of local butterflies and their movement and dispersion in the landscape, allowing the maintenance of metacommunity dynamics in this unique and threatened area in the Colombian Andes. Thus, it is crucial to increase knowledge on small-scale research by evaluating the modification of habitats and their associated fauna as a useful tool for future management and conservation purposes (Bellaver et al. 2022). According to Bonebrake et al. (2010), the development of a predictive theory of the impacts of habitat loss on tropical butterfly diversity requires more research on specific biogeography patterns, demographics and life history strategies, and interactions between species. Those are still imperative in high Andean butterflies research, in which long-term data recording and information availability enable robust data analyze. Additionally, the adoption and continuous refinement of standardized monitoring protocols are necessary to effectively disentangle the consequences of the impacts of the Anthropocene and climate change on butterflies inhabiting high elevational zones in the tropics.
Data availability
All data are available in the article body or supporting information.
References
Adams MJ (1985) Speciation in the pronophiline butterflies (Satyridae) of the Northern Andes. J Res Lepidoptera Supplement, 33–49
Álvarez CF, Clavijo-Giraldo A, Uribe SI, Pyrcz TW, Iserhard CA, Freitas AVL, Marín MA (2021) Sampling performance of bait traps in high Andean fruit-feeding butterflies. Neotrop Biodivers 7:507–513. https://doi.org/10.1080/23766808.2021.2004802
Álvarez-Hincapié CF, Clavijo A, Rojas H, Uribe SI, Pyrcz TW, Marín MA (2017) Aporte Del área de influencia del páramo de belmira (Santa Inés) a la diversidad regional de Pronophilina (Lepidoptera: Satyrinae) Del Norte De Los Andes. Rev Mex Biodivers 88:402–409. https://doi.org/10.1016/j.rmb.2017.03.007
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecol 26:32–46. https://doi.org/10.1111/j.1442-9993.2001.01070.pp.x
Armenteras D, Gast F, Villareal H (2003) Andean forest fragmentation and the representativeness of protected natural areas in the eastern Andes, Colombia. Biol Conserv 113:245–256. https://doi.org/10.1016/S0006-3207(02)00359-2
Barlow J, Overal WL, Araujo IS, Gardner TA, Peres CA (2007) The value of primary, secondary and plantation forests for fruit-feeding butterflies in the Brazilian Amazon. J Appl Ecol 44:1001–1012. https://doi.org/10.1111/j.1365-2664.2007.01347.x
Barlow J, Araujo IS, Overal WL, Gardner TA, Mendes FD, Lake IR, Peres CA (2008) Diversity and composition of fruit-feeding butterflies in tropical Eucalyptus plantations. Biodivers Conserv 17:1089–1104. https://doi.org/10.1007/s10531-007-9240-0
Bax V, Francesconi W, Delgado A (2019) Land-use conflicts between biodiversity conservation and extractive industries in the Peruvian Andes. J Environl Manag 232:1028–1036. https://doi.org/10.1016/j.jenvman.2018.12.016
Bellaver J, Romanowski HP, Richter A, Iserhard CA (2022) Living on the edge: the use of fruit-feeding butterflies to evaluate edge effect on subtropical assemblages. Austral Ecol 48(2):217–232. https://doi.org/10.1111/aec.13261
Blair RB, Launer AE (1997) Butterfly diversity and human land use: species assemblages along an urban gradient. Biol Conserv 80(1):113–125. https://doi.org/10.1016/S0006-3207(96)00056-0
Bonebrake TC, Ponisio LC, Boggs CL, Ehrlich PR (2010) More than just indicators: a review of tropical butterfly ecology and conservation. Biol Conserv 143:1831–1841. https://doi.org/10.1016/j.biocon.2010.04.044
Brehm G, Homeier J, Fiedler (2003) Beta diversity of geometrid moths (Lepidoptera: Geometridae) in an Andean montane rainforest. Divers Distrib 9(5):351–366. https://doi.org/10.1046/j.1472-4642.2003.00023.x
Burghardt KT, Tallamy DW, Philips C, Shropshire KJ (2010) Non-native plants reduce abundance, richness, and host specialization in lepidopteran communities. Ecosphere 1:art11. https://doi.org/10.1890/ES10-00032.1
Caldas A, Robbins RK (2003) Modified Pollard transects for assessing tropical butterfly abundance and diversity. Biol Conserv 110:211–219. https://doi.org/10.1016/S0006-3207(02)00190-8
Casas-Pinilla LC, Iserhard CA, Richter A, Gawlinski K, Cavalheiro LBD, Romanowski H, Kaminski L (2022) Different-aged Pinus afforestation does not support typical Atlantic Forest fruit-feeding butterfly assemblages. For Ecol Manag 518:120279. https://doi.org/10.1016/j.foreco.2022.120279
CEPF (2021) Ecosystem profile: Tropical Andes Biodiversity Hotspot. Critical Ecosystem Partnership Fund, Arlington USA
Chao A, Jost L (2012) Coverage-based rarefaction and extrapolation: standardizing samples by completeness rather than size. Ecology 93:2533–2547. https://doi.org/10.1890/11-1952.1
Chao A, Shen T-J (2003) Nonparametric estimation of Shannon’s index of diversity when there are unseen species in sample. Environ Ecol Stat 10:429–443. https://doi.org/10.1023/A:1026096204727
Chao A, Gotelli NJ, Hsieh TC, Sander EL, Ma KH, Colwell RK, Ellison AM (2014) Rarefaction and extrapolation with Hill numbers: a framework for sampling and estimation in species diversity studies. Ecol Monogr 84:45–67. https://doi.org/10.1890/13-0133.1
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Aust J Ecol 18:117–143. https://doi.org/10.1111/j.1442-9993.1993.tb00438.x
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.r-project.org/
Cuatrecasas J (1958) Aspectos De La vegetación natural de Colombia. Rev Acad Colomb Cienc Exactas Fis Nat 10:221–264
Dennis RLH, Dapporto L, Dover JW (2014) Ten years of the resource-based habitat paradigm: the biotope-habitat issue and implications for conserving butterfly diversity. J Insect Biodivers 2:1. https://doi.org/10.12976/jib/2014.2.8
Dover JW, Rescia A, Fungariño S, Fairburn J, Carey P, Lunt P, Dennis RLH, Dover CJ (2010) Can hay harvesting detrimentally affect adult butterfly abundance? J Insect Conserv 14:413–418. https://doi.org/10.1007/s10841-010-9267-5
Dufrêne M, Legendre P (1997) Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecol Monogr 67:345–366. https://doi.org/10.2307/2963459
Etter A, van Wyngaarden W (2000) Patterns of landscape transformation in Colombia, with emphasis in the Andean region. Ambio 29:432–439. https://doi.org/10.1579/0044-7447-29.7.432
Feeley KJ, Hurtado J, Saatchi S et al (2013) Compositional shifts in Costa Rican forests due to climate-driven species migrations. Glob Change Biol 19:3472–3480. https://doi.org/10.1111/gcb.12300
Ferreira FC, Petrere M (2008) Comments about some species abundance patterns: classic, neutral, and niche partitioning models. Braz J Biol 68:1003–1012. https://doi.org/10.1590/s1519-69842008000500008
Freitas AVL, Iserhard CA, Santos JP, Carreira JYO, Ribeiro DB, Alves DHA, Rosa AHB, Marini-Filho OJ, Accacio GM, Uehara-Prado M (2014) Studies with butterfly bait traps: an overview. Rev Colomb Entomol 40:209–218
Hammer Ø, Harper DAT, Ryan PD PAST: Paleontological statistics software package for education and data analysis. Palaeontol Electron 4(1):4,9pp., Harvey JA, Tougeron K, Gols R et al (2001) (2023) Scientists’ warning on climate change and insects. Ecological Monographs 93:e1553. https://doi.org/10.1002/ecm.1553
Herzog SK, Kattan GH (2011) Patterns of diversity and endemism in the birds of the Tropical Andes. In: Herzog SK, Martinez R, Jorgensen PM, Tiessen H (ed) Climate change and biodiversity in the tropical Andes. Inter-American Institute for Global Change Research (IAI) and Scientific Committee on Problems of the Environment (SCOPE), pp 245–259
Hill MO (1973) Diversity and evenness: a unifying notation and its consequences. Ecology 54(2):427–432. https://doi.org/10.2307/1934352
Hsieh TC, Ma KH, Chao A (2020) iNEXT: iNterpolation and EXTrapolation for species diversity. R package version 2.0.20. https://cran.r-project.org/web/packages/iNEXT/index.html
Hurlbert SH (1971) The nonconcept of species diversity: a critique and alternative parameters. Ecology 52:577–586. https://doi.org/10.2307/1934145
Iserhard CA, Romanowski HP, Richter A, Mendonça MDS Jr (2017) Monitoring temporal variation to assess changes in the structure of subtropical Atlantic Forest butterfly communities. Environ Entomol 46:804–813. https://doi.org/10.1093/ee/nvx115
Iserhard CA, Duarte L, Seraphim N, Freitas AVL (2019) How urbanization affects multiple dimensions of biodiversity in tropical butterfly assemblages. Biodivers Conserv 28:621–638. https://doi.org/10.1007/s10531-018-1678-8
Jost L (2006) Entropy and diversity. Oikos 113:363–375. https://doi.org/10.1111/j.2006.0030-1299.14714.x
Kraft NJ, Cornwell WK, Webb CO, Ackerly DD (2007) Trait evolution, community assembly, and the phylogenetic structure of ecological communities. Am Nat 170:271–283. https://doi.org/10.1086/519400
Lorandi S, Halinski R, Mustin K, Iserhard CA (2023) Are there differences in the diversity of bees between organic and conventional agroecosystems in the Pampa biome? J Apicult Res 62(2):250–262. https://doi.org/10.1080/00218839.2021.1888524
Lourenço GM, Soares GR, Santos TP, Dáttilo W, Freitas AVL, Ribeiro SP (2019) Equal but different: natural ecotones are dissimilar to anthropic edges. PLoS ONE 14(3):e0213008. https://doi.org/10.1371/journal.pone.0213008
Mahecha O, Garlacz R, Andrade MG, Prieto C, Pyrcz TW (2019) Island biogeography in continental areas: inferring dispersal based on distributional patterns of Pronophilina butterflies (Nymphalidae: Satyrinae) in the north Andean massifs. Rev Mex Biodivers 90:e902796. https://doi.org/10.22201/ib.20078706e.2019.90.2796
Mair L, Hill JK, Fox R, Botham M, Brereton T, Thomas CD (2014) Abundance changes and habitat availability drive species’ responses to climate change. Nat Clim Change 4:127–131. https://doi.org/10.1038/nclimate2086
Marín MA, Álvarez CF, Giraldo CE, Pyrcz TW, Uribe SI, Vila R (2014) Mariposas en un bosque de niebla andino periurbano en El valle de Aburrá, Colombia. Rev Mex Biodivers 85:200–208. https://doi.org/10.7550/rmb.36605
Marín MA, Giraldo CE, Marín AL, Álvarez CF, Pyrcz TW (2015) Differences in butterfly (Nymphalidae) diversity between hillsides and hilltop forest patches in the Northern Andes. Stud Neotrop Fauna Environ 50(3):194–203. https://doi.org/10.1080/01650521.2015.1099379
Matsumoto K (2015) Habitat specificity of butterflies along urban environmental gradients in Tama city. Tokyo Entomol Sci 18:509–518. https://doi.org/10.1111/ens.12146
McGill BJ, Etienne RS, Gray JS, Alonso D, Anderson MJ, Benecha HK, Dornelas M, Enquist BJ, Green JL, He F, Hurlbert AH, Magurran AE, Marquet PA, Maurer BA, Ostling A, Soykan CU, Ugland KI, White EP (2007) Species abundance distributions: moving beyond single prediction theories to integration within an ecological framework. Ecol Lett 10:995–1015. https://doi.org/10.1111/j.1461-0248.2007.01094.x
Melo DHA, Filgueiras BKC, Iserhard CA, Iannuzzi L, Freitas AVL, Leal IR Effect of habitat loss and fragmentation on fruit-feeding butterflies in the Brazilian Atlantic Forest. Can J Zool 97(7):588–596., de Aráuz M los Á, Gobbi M, Barragán Á (2019) (2016) Climate warming effects in the tropical Andes: first evidence for upslope shifts of Carabidae (Coleoptera) in Ecuador. Insect Conserv Divers 9:342–350. https://doi.org/10.1111/icad.12173
Moguel-Cárdenas LI, León‐Cortés JL, Rodríguez‐Aguilar O, Castillo‐Vera A, Islebe GA (2024) Climate‐driven change and conservation of threatened satyrine butterflies in cloud forests of southern Mexico. J Insect Conserv. https://doi.org/10.1007/s10841-024-00553-8
Moret P, Aráuz MA, Gobbi M, Barragán A (2016) Climate warming effects in the tropical Andes: first evidence for upslope shifts of Carabidae (Coleoptera) in Ecuador. Insect Conserv Diver 9: 342–350.https://doi.org/10.1111/icad.12173
Mtui DT, Ogutu JO, Okick RE, Newmark WD (2022) Elevational distribution of montane afrotropical butterflies is influenced by seasonality and habitat structure. PLoS ONE 17(7):e0270769. https://doi.org/10.1371/journal.pone.0270769
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Szoecs E, Wagner H (2019) vegan: Community Ecology Package. R package version 2.5-6. https://cran.r-project.org/package=vegan
Otero J, Onaindia M (2009) Landscape structure and live fences in Andes Colombian agrosystems: Upper basin of the Cane-Iguaque River. Rev Biol Trop 57:1183–1192. https://doi.org/10.15517/rbt.v57i4.5455
Peyre G, Lenoir J, Karger DN, Gomez M, Gonzalez A, Broennimann O, Guisan A (2020) The fate of páramo plant assemblages in the sky islands of the Northern Andes. J Veg Sci 31:967–980. https://doi.org/10.1111/jvs.12898
Pyrcz TW, Wojtusiak J, Garlacz R (2009) Diversity and distribution patterns of Pronophilina butterflies (Lepidoptera: Nymphalidae: Satyrinae) along an altitudinal transect in north-western Ecuador. Neotrop Entomol 38(6):716–726. https://doi.org/10.1590/S1519-566X2009000600003
Pyrcz TW, Willmott KR, Garlacz R, Boyer P, Gareca Y (2014) Latitudinal gradient and spatial covariance in species richness of tropical Lepidoptera in the Andes. Insect Conserv Diver 7:355–364. https://doi.org/10.1111/icad.12058
Pyrcz TW, Clavijo A, Uribe SI, Marín MA, Álvarez CF, Zubek A (2016) Páramo De Belmira as an important centre of endemism in the northern Colombian Andes: new evidence from Pronophilina butterflies (Lepidoptera: Nymphalidae, Satyrinae, Satyrini). Zootaxa 4179:77–102. https://doi.org/10.11646/zootaxa.4179.1.3
Rabl D, Gottsberger B, Brehm G, Hofhansl F, Fiedler K (2020) Moth assemblages in Costa Rica rain forest mirror small-scale topographic heterogeneity. Biotropica 52:288–301. https://doi.org/10.1111/btp.12677
Rangel-Ch JO (2005) La Biodiversidad De Colombia. Palimpsesto 5:292–304
Ribeiro DB, Freitas AVL (2012) The effect of reduced-impact logging on fruit-feeding butterflies in Central Amazon, Brazil. J Insect Conserv 16:733–744. https://doi.org/10.1007/s10841-012-9458-3
Ribeiro DB, Batista R, Prado PI, Brown KS, Freitas AVL (2012) The importance of small scales to the fruit-feeding butterfly assemblages in a fragmented landscape. Biodivers Conserv 21:811–827. https://doi.org/10.1007/s10531-011-0222-x
Rödder D, Schmitt T, Gros P, Ulrich W, Habel JC (2021) Climate change drives mountain butterflies towards the summits. Sci Rep 11:14382. https://doi.org/10.1038/s41598-021-93826-0
Santos JP, Iserhard CA, Teixeira MO, Romanowski HP (2011) Fruit-feeding butterflies guide of subtropical Atlantic Forest and Araucaria Moist Forest in State of Rio Grande do sul, Brazil. Biota Neotrop 11(3):256–274. https://doi.org/10.1590/S1676-06032011000300022
Shapiro A (1992) Why are there so few butterflies in the high Andes? J Res Lepidoptera 31:35–56
Spaniol RL, Duarte L, Mendonça MDS Jr, Iserhard CA (2019) Combining functional traits and phylogeny to disentangling amazonian butterfly assemblages on anthropogenic gradients. Ecosphere 10:1–15. https://doi.org/10.1002/ecs2.2837
Tobón C (2021) Ecohydrology of Tropical Andean Cloud Forests. In ‘The Andean Cloud Forest’. (Ed R. W. Myster.) pp. 61–87. (Springer Nature: Switzerland AG.) https://doi.org/10.1007/978-3-030-57344-7_4
Uehara-Prado M, Freitas AVL (2009) The effect of rainforest fragmentation on species diversity and mimicry ring composition of ithomiine butterflies. Insect Conserv Divers 2:23–28. https://doi.org/10.1111/j.1752-4598.2008.00025.x
Uehara-Prado M, Brown KS, Freitas AVL (2007) Species richness, composition and abundance of fruit-feeding butterflies in the Brazilian Atlantic Forest: comparison between a fragmented and a continuous landscape. Global Ecol Biogeogr 16:43–54. https://doi.org/10.1111/J.1466-8238.2006.00267.X
van der Hammen T (1974) The pleistocene changes of vegetation and climate in tropical South America. J Biogeogr 1:3–26. https://doi.org/10.2307/3038066
Venables WN, Ripley BD (2002) Modern Applied statistics with S, 4th edn. Springer, New York.)
Whittaker RH (1965) Dominance and diversity in land plant communities. Science 147:250–260. https://doi.org/10.1126/science.147.3655.250
Williamson M, Gaston KJ (2005) The lognormal distribution is not an appropriate null hypothesis for the species-abundance distribution. J Anim Ecol 74:409–422. https://doi.org/10.1111/j.1365-2656.2005.00936.x
Wilson JB (1991) Methods for fitting dominance/diversity curves. J Veg Sci 2:35–46. https://doi.org/10.2307/3235896
Acknowledgements
We would like to thank the local community of Belmira, in particular Hector Rojas for the field support, to Francisco Restrepo, Estiben Galeano, Johanna M. Osorio, and Jaime Duque. Tomasz Pyrcz and Alejandra Clavijo for the taxonomic support, and the GSM group of the Universidad Nacional de Colombia Sede Medellín. We are grateful to two anonymous reviewers for critically reviewing and valuable contributions to the manuscript. In accordance with the environmental legislation of Colombia, this work was done under the license number 4 of May 7th 2011 and the resolution 503 of May 24th 2013 from the National Agency for Environmental Licenses (Agencia Nacional de Licencias Ambientales – ANLA, Colombia).
Funding
CFA was supported by a Doctoral grant from Departamento Administrativo de Ciencia, Tecnología e Innovación (Colciencias - Colombia) [call 528/2011]. AVLF thanks the Brazilian Research Council – CNPq [563332/2010-7, 303834/2015-3 and 304291/2020-0], Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP – Brazil) [grant 2011/50225-3 and 2021/03868-8], Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES - Brazil) [Finance code 001] and National Science Foundation (NSF - USA) [DEB-1256742]. MAM received a postdoctoral fellowship from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP -Brazil) [grant 2018/11910-1]. CAI is member of the National Institutes for Science and Technology (INCT - Brazil) in Ecology, Evolution and Biodiversity Conservation, supported by [MCTIC/CNPq proc. 465610/2014-5] and [FAPEG proc. 201810267000023].
Open Access funding provided by Colombia Consortium
Author information
Authors and Affiliations
Contributions
Conceptualization: [Carlos Federico Álvarez, André Victor Lucci Freitas, Cristiano Agra Iserhard, Mario Alejandro Marín]; Data curation: [Carlos Federico Álvarez, Carlos Eduardo Giraldo, Mario Alejandro Marín]; Formal analysis: [Carlos Eduardo Giraldo, Mario Alejandro Marín]; Funding acquisition: [Carlos Federico Álvarez, Sandra Inés Uribe]; Investigation: [Carlos Federico Álvarez, Carlos Eduardo Giraldo, Mario Alejandro Marín]; Methodology: [Carlos Federico Álvarez, André Victor Lucci Freitas, Cristiano Agra Iserhard, Mario Alejandro Marín]; Project administration: [Carlos Federico Álvarez, Sandra Inés Uribe]; Resourses: [Carlos Federico Álvarez, Sandra Inés Uribe]; Supervision: [André Victor Lucci Freitas, Cristiano Agra Iserhard, Sandra Inés Uribe]; Validation: [Carlos Federico Álvarez, André Victor Lucci Freitas, Cristiano Agra Iserhard, Mario Alejandro Marín]; Visualization: [Mario Alejandro Marín]; Writing – original draft: [Mario Alejandro Marín]; Writing – review & editing [ Carlos Federico Álvarez, André Victor Lucci Freitas, Cristiano Agra Iserhard, Carlos Eduardo Giraldo, Sandra Inés Uribe, Mario Alejandro Marín].
Corresponding author
Ethics declarations
Conflict of interest
The work was done under the license for specimen collection number 4 of May 7th 2011 and the resolution 503 of May 24th 2013 from the National Agency for Environmental Licenses (Agencia Nacional de Licencias Ambientales – ANLA, Colombia). The authors declare no conflicts of interest regarding this study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Álvarez, C.F., Freitas, A.V.L., Iserhard, C.A. et al. Structure of the assemblage of fruit-feeding butterflies in a high Andean anthropogenic landscape. J Insect Conserv (2024). https://doi.org/10.1007/s10841-024-00600-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10841-024-00600-4