Abstract
Aim
Schizomids are one of the less-known arachnid groups in terms of their natural history and ecology. However, due to their remarkable short-range endemic distribution, they may be vulnerable to climate change and habitat loss. In Colombia, although the national IUCN red list of threatened invertebrates has categorized species of schizomids as vulnerable (VU), this assessment was based on expert criteria. Therefore, information about the ecology of schizomids is critical for a more accurate reassessment of their conservation status. In this study, we describe the habitat of two species of Surazomus in endangered Andean tropical forests of Colombia after a sampling effort of 15.12 m2 (n = 168 soil samples) and the collection of 6999 soil fauna individuals from the samples. We analyzed soil fauna communities associated with schizomids as well as different forest and environmental variables from permanent plots installed a decade ago in the Sabana de Bogotá region. Detailed information on climate, plant communities, and forest structure was obtained from these plots. Thus, we provide the first comprehensive habitat description of schizomids including both above- and belowground compartments. We found that each species lives in specific habitats with different soil fauna communities, suggesting a potential association between geographical fidelity and habitat conditions. This result could indicate that schizomids are highly sensitive to dramatic environmental changes, such as those experienced in the Andean region of Colombia.
Implications for insect conservation
Our study is valuable for the future reassessment of the conservation status of schizomids in the country, particularly considering that the previous categorization was based on expert criteria. Since habitat conditions and soil fauna communities are species-specific, schizomids could be disproportionately vulnerable to climate change and human disturbances in the Colombian Andes.
Similar content being viewed by others
Introduction
The order Schizomida, commonly known as short-tailed whip scorpions, is one of the smaller arachnid groups (Harvey 2003) and currently comprises 386 species (14 fossils), 79 genera (eight fossils), and three families (one fossil) (World Schizomida Catalog 2022). Schizomids are small predators (2.0–12.5 mm) widely distributed in tropical and subtropical areas around the World. They are frequently found in different habitats, including forests, caves, and human-dominated landscapes such as botanical gardens, greenhouses, and crops. These habitats offer multiple microhabitats, such as leaf-litter, soil, termite nests, rock crevices and rotten logs, which provide the necessary resources for maintaining their populations (Reddell and Cokendolpher 1995).
The vast majority of schizomids show remarkable geographical fidelity (Clouse et al. 2017) and are only known from single localities (type localities) or a few close localities. Thus, most of them have been considered short-range endemic taxa, a category for species distributed in < 10,000 km2 (Harvey 2002). In consequence, schizomids may be especially vulnerable to climate change and habitat loss. Although some progress has been made regarding their systematics (e.g., Monjaraz-Ruedas et al. 2016; Clouse et al. 2017), paleontology (e.g., Magnussen et al. 2022), and morphology (e.g., Kallal et al. 2022), little is known about the natural history and ecology of these arachnids. For example, the behavior has been studied in only three species (Sturm 1958, 1973; Kraus and Beck 1967; Oliveira and Ferreira 2014), a handful of predators and preys of schizomids have been reported (Armas and Melic 2015; Armas and Moreno-González 2021), and the knowledge on reproductive biology is restricted to isolated observations of brooding (Giribet and Moreno-González 2021).
Our understanding about the ecology of South American schizomids is particularly reduced, despite occupying several ecosystems from sea level to upper Andean montane forests (Armas 2010). Available ecological contributions have mainly focused on the abundance and phenology of Amazonian schizomid communities (Adis et al. 1999, 2001). Nonetheless, there are no studies dealing with the co-occurrence of schizomids and other edaphic invertebrate communities, as well as the variables that modulate their habitat preference. In fact, most of the habitat characterizations available of schizomids consist of simple descriptions without a methodology to evaluate the biotic and abiotic conditions where schizomids live.
In turn, this lack of knowledge on the ecology of schizomids hampers the conservation efforts to protect them, which is particularly critical in the Andes of Northern South America. This region has experienced a legacy of large-scale deforestation that has triggered a pervasive habitat fragmentation, leaving isolated patches of old-growth forests (Etter et al. 2008). This is particularly evident in the Sabana de Bogotá region, where less than 30% of its original forest cover remains (Etter et al. 2021), and where an endemic schizomid species, Surazomus sturmi, is distributed (Kraus 1957). Due to this pervasive habitat loss, the national IUCN red list of threatened invertebrates has categorized S. sturmi as vulnerable (VU) (Flórez and Cepeda 2007). Nonetheless, this assessment was based on expert criteria and, therefore, information about the ecology of this species is critical for a more accurate assessment of its conservation status. Understanding the ecology of schizomids is also crucial for recommending management and land use strategies that support the conservation of these organisms. On the other hand, very few sampling efforts have been conducted in the peri-urban forest of the Sabana de Bogotá region, where other undescribed species of Surazomus are distributed.
In this paper, we characterized the habitat of two threatened Surazomus species from the Eastern Andes of Colombia, thus providing new information about environmental conditions, co-occurrence with other soil fauna, and forest attributes derived from long-term monitoring of permanent plots.
Methods
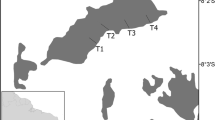
Our study took place in the Eastern Colombian Andes along a successional forest gradient in the Cundiboyacense high plains at elevations ranging from 2685 to 3140 m. The Andean region covers 24.5% of the Colombian territory (Etter and Wyngaarden 2000) and is the most populated region in the country, accounting for 70% of total population (DANE 2005). In this region, a set of 36 20 m × 20 m and eight 50 m × 50 m permanent plots were established starting in 2013 in five municipalities (Guasca, Guatavita, Tabio, San Francisco, and Soacha) and in peri-urban areas around Bogotá (Torca), the capital of Colombia, to monitor carbon cycling and biodiversity (both functional and taxonomic) in the long-term (Fig. 1). The plots are placed on private properties/reserves and encompass secondary and mature forests based on their structural attributes (i.e., basal area, tree height, tree density) and species composition (see Hurtado-M et al. 2021; Castillo-Figueroa et al. 2023). We established a successional gradient along the plots using the aboveground biomass from allometric equations as a proxy of forest recovery (Castillo-Figueroa et al. 2023).
Species of Surazomus and their habitats in the Sabana de Bogotá region (Cundinamarca, Colombia). a Forest in Tabio; b female of Surazomus sp. over Dapnhopsis caracasana litter; c forest in Torca (Bogotá); d female of Surazomus sturmi over Clusia multiflora litter. The yellow circles on the map indicate the locations of the 14 permanent plots where soil fauna sampling took place. (Color figure online)
We sampled soil fauna communities in surface litter and in 30 cm × 30 cm × 5 cm depth samples in soils in 14 of the plots during four climatic seasons of 2022 (dry: Jan–Feb; wet: Apr–May; moderately dry: Jul–Aug; wet: Oct–Nov), as a part of a litter decomposition experiment. In this experiment, three litterbeds with a minimal distance of 5 m between them were installed per plot for a total of 42 experimental units (Castillo-Figueroa et al. in prep.). Three soil samples were collected near the litterbeds for each plot between 8:00 and 12:00, resulting in a total of 42 samples across the 14 plots and 168 samples spanning the four climatic seasons. We stored litter and soil samples in separate plastic bags to distinguish soil fauna from: (1) leaf-litter and (2) soil depth at 0–5 cm. In the lab, soil fauna was manually extracted and preserved in 70% ethanol. Taxonomic identification was carried out with the help of experts, original descriptions, and with the existing identification keys for the region to achieve the best taxonomic resolution possible. We also conducted field trips in all the sites, during March 2023, to manually search for schizomids on the forest floor. In this field campaigns, two researchers (DCF and CCA) were looking for schizomids during 2 h in all the potential microhabitats they can inhabit within the 20 m × 20 m permanent plots such as leaf-litter, rock crevices, rotten logs, and soil. This comprised a total surface area of 5600 m2 and 56 h of active searching. The individuals that we found in these field trips and in the litter and soil sampling, were used to analyze the relationships between environmental conditions and the presence of schizomids.
To characterize the microhabitat of schizomids, we installed TMS-4 dataloggers (TOMST, Czech Republic) in the center of each plot. We recorded temperature 15 cm above the soil surface, at the soil level, and at 8 cm depth, as well as soil volumetric water content every 15 min (Wild et al. 2019). To describe forest structure, we quantified canopy openness and Leaf Area Index (LAI), by taking hemispherical photographs (Canon Inc. EW-77 Fisheye Zoom Lens EF 8–15 mm 1:4 (Japan), which were then processed with Gap Light Analyzer (GLA version 2.0, https://www.caryinstitute.org/). Finally, litter depth was averaged from five random soil points within each sampling unit using a digital caliper (0.1 mm).
We performed a non-metric multidimensional scaling (NMDS) with the edaphic communities associated to the litter and soil samples where the species of Surazomus were found. This was complemented with an Analysis of Similarity (ANOSIM) based on 9999 permutations and a Similarity Percentage breakdown analysis (SIMPER) (Clarke 1993). All three analyses were performed using Bray–Curtis distance. To describe the habitat of Surazomus, we did a Principal Component Analysis (PCA) with the environmental variables associated to the samples where individuals of Surazomus were collected, including the manual samplings in the ground. In this PCA, we also incorporated pre-existing data from the permanent plots such as litter production, soil pH, bulk density, and litter decay (Tables S1, S2). Initially, we had 14 variables obtained from the permanent plots. However, we refined our selection by excluding highly correlated variables (rho > 0.7) and those considered less informative regarding the environmental and structural forests characteristics (Fig. S1). As a result, we performed a PCA with eight highly informative variables, providing a more accurate representation of the forest structure, edaphic characteristics, and climatic conditions associated with each species of Surazomus. In this PCA, data points were scaled by 1/√dk and the biplot eigenvectors by √dk according to the correlation biplot of Legendre and Legendre (1998). To assess the suitability of PCA as a method for dimensionality reduction, Bartlett’s test of sphericity was used (Bartlett 1951). This test evaluates the null hypothesis that the variables are not interrelated and, therefore, may not be suitable for a PCA. Rejection of this null hypothesis indicates a correlation among variables, thereby supporting the assumption required for PCA to be effective in capturing underlying patterns and reducing dimensionality. Lastly, we did pairwise comparisons in the microhabitat characteristics between the sites where the species of Surazomus were found through a t test. All the statistical analyses were performed in PAST 4.14 (Hammer et al. 2001) and JASP 0.18.1.0 (JASP TEAM 2023).
Results and discussion
In three distinct seasons (dry: Jan–Feb, wet: Apr–Mar, wet: Oct–Nov), we found two species of Surazomus in the Sabana de Bogotá region: Surazomus sturmi (Kraus 1957) in Torca and an undescribed species of Surazomus in Tabio (Moreno-González in prep.). Both species could be readily identified based on the shape of the male flagella and female spermathecae, which are commonly used morphological characters for species distinction in Schizomida (Reddell and Cokendolpher 1995). After a sampling effort of 15.12 m2 and 168 samples and the collection of 6999 soil fauna individuals from the samples, we found only five individuals of S. sturmi in Torca (4♀, 1♂) and six individuals of Surazomus sp. in Tabio (4♀, 2♂) (Fig. 1), representing the 0.16% of the total soil fauna community. Despite this, within the nine samples where schizomids were present, their relative abundance was 3.7% in Torca and 3.2% in Tabio (Table S3). The sex ratio of 4:1 females to males in Torca and 2:1 in Tabio is consistent with findings reported for other species of Surazomus (Adis et al. 1999, 2001), however, it is important to exercise caution when interpreting these sex ratios due to the relatively low number of schizomids collected in this study.
We found that habitat conditions and soil fauna communities are species-specific, which is consistent with the short-range distribution of schizomids (Harvey 2002). In the case of S. sturmi, our results show that they can be found mainly in old-growth forests with large trees that shed vast amounts of litter into the soil (Fig. 2a, Tables S1, S2). These forests are dominated by plant species with conservative leaf traits such as Weinmannia tomentosa L. f., Clusia multiflora Kunth, Cavendishia nitida (Kunth) A.C. Sm., and other Ericaceae species with thick leaves that decompose slowly. High litter production and slow decay rates likely explain the high litter depth on the forest floor and the thicker soil organic horizon (Figs. 2a, S2, Tables S1, S2). On the other hand, individuals of Surazomus sp. from Tabio were found in both old-growth and secondary forests, with higher soil temperatures (t = 236.273, P < 0.001, Cohen’s d = 1.193), decay rates (t = 2.491, P = 0.014, Cohen’s d = 0.349), and soil moisture (t = 69.100, P < 0.001, Cohen’s d = 0.349) than in Torca (Fig. 1, Table S1). Forests in Tabio are dominated by more acquisitive plant species such as Cedrela montana Moritz ex Turcz., Daphnopsis caracasana Meisn., Miconia squamulosa (Sm.) Triana and Croton bogotanus Cuatrec.
Habitat characteristics and soil fauna communities associated to each Surazomus species. a Principal Component Analysis (PCA) of 14 Surazomus sampled and eight environmental characteristics associated to them (green arrows) including air temperature (°C), soil moisture (%), soil pH, bulk density (g/cm3), canopy openness (%), litter depth (cm), litter decay (% mass loss), and litter production (Ton C ha−1 year−1). The first two principal components accumulate 81.92% of total variation. Barlett’s sphericity test indicates strong relationships among the variables (Chi-square = 235.24, df = 28, P < 0.0001), supporting their suitability for the PCA. Loading plots are depicted in Fig. S2. b Non-metric multidimensional scaling (NMDS) of soil fauna communities where Surazomus were found. The number of dimensions was set to 3 (k = 3), however we represent dissimilarity in a two-dimensional space to highlight differences between soil fauna communities associated to each Surazomus species. Stress = 0.15. In both PCA and NMDS, crimson squares depict Surazomus sp. from Tabio, while blue dots correspond to S. sturmi from Torca. In the NMDS, squares and dots correspond to nine soil samples (five in Tabio and four in Torca), in which we found 11 individuals of Surazomus species (six in Tabio and five in Torca). (Color figure online)
Interestingly, out of the 14 individuals collected (8♀, 3♂ from soil samples and 3♀ from manual samplings), 10 were found in the soil layer which was the microsite with higher mean temperature and lower variability (X = 12.39 °C, CV = 0.04) than surface (X = 12.06 °C, CV = 0.12) and air (X = 11.54 °C, CV = 0.22). Considering that low temperatures in high Andean forests are common, especially at dawn, Surazomus may select warmer habitats at a certain depth in the soil, avoiding the exposure to cold air temperatures. This result is in agreement with Adis et al. (1999), who found a positive relation between temperature and abundance of S. brasiliensis in late successional lowland tropical forests. This suggests that Surazomus species actively search for microhabitats that improve thermoregulation, thus avoiding low thermal conditions of their environment. However, Adis et al. (2001) reported a decrease in the abundance of S. mirim and S. rodriguesi in the Amazon with increasing temperature in early successional forests, where atmospheric temperature is warm because of high canopy openness and solar radiation exposure. Due to the low number of individuals in each sample (1 to 2), we could not establish a correlation between temperature and schizomid abundance, even though 70% of the individuals were found in the soil layer, which displayed a higher mean temperature and higher thermal stability. Despite the very low local abundance of these arachnids, further studies are needed to determine whether mean temperature and its variability act as limiting factors in schizomid microhabitat preference.
The NMDS (Figs. 2b, S3) and the ANOSIM (P = 0.008, R = 0.85) indicated that Surazomus. sp. from Tabio and S. sturmi from Torca were associated with different soil fauna communities. Although some taxonomic groups were present only in one site, millipedes and mites were the most dominant groups in both sites (Tables S3, S4). According to the SIMPER analysis, species of millipedes (Trichopolydesmidae, Pseudonannolenidae), mites (Oribatida), isopods (Scleropactidae), pseudoscorpions (Ideobisium sp.), and diplurans (Campodeidae) accounted for 42.26% of the dissimilarity between the two edaphic communities (Table S4). It has been suggested that schizomids may prey on millipedes, cockroaches, symphylans, springtails, small worms, isopods, and even other schizomids (Humphreys et al.1989; Armas and Melic 2015); however, dietary analyses are required to confirm this statement. Centipedes, pseudoscorpions and spiders are also listed as potential natural enemies of schizomids (Armas and Moreno-González 2021), and they were found in the soil fauna communities but in low proportions (Tables S3, S4). Further research should analyze the interactions between these endangered schizomid species and other soil animals and its implications on soil food webs.
Schizomids are still understudied, and our basic understanding of their ecology remains limited. Besides our study, there are no detailed descriptions of their habitats including both above- and belowground compartments. Despite the limited number of individuals collected in this study, our findings suggest that the short-range distribution of schizomids may be associated with specific habitat conditions where they live, which could render them highly sensitive to dramatic environmental changes. However, further studies with larger sampling areas that encompass more sites and individuals collected should address this issue. We hope this study could be useful in the reassessment of the conservation status of schizomids in Colombia and encourages more ecological investigations on these poorly studied soil arachnids.
Data availability
The data and supplementary material that support the findings of this study are openly available in Open Science Framework repository at https://osf.io/y2qfs/ (Castillo-Figueroa 2023).
References
Adis JU, Cokendolpher JC, Reddell JR, Rodrigues JMG (2001) Abundance and phenology of Schizomida (Arachnida) from a secondary upland forest in Central Amazonia. Rev Suisse Zool 108:879–889. https://doi.org/10.5962/bhl.part.80166
Armas LF (2010) Schizomida de Sudamerica (Chelicerata: Arachnida). Boletín De La SEA 46:203–234
Armas LF, Melic A (2015) Orden Schizomida. Revista Idea-SEA 21:1–6
Armas LF, Moreno-González JA (2021) Predators of the short-tailed whipscorpions (Schizomida): are they so few? Rev Iber Aracnol 38:189–190
Bartlett MS (1951) The effect of standardization on a Chi-square approximation in factor analysis. Biometrika 38:337–344. https://doi.org/10.2307/2332580
Castillo-Figueroa D (2023) Supplemental material for “Habitat of two threatened short-tailed whip-scorpions (Arachnida: Schizomida: Hubbardiidae) in the Andes of Northern South America.” https://osf.io/y2qfs/
Castillo-Figueroa D, González-Melo A, Posada JM (2023) Wood density is related to aboveground biomass and productivity along a successional gradient in upper Andean tropical forests. Front Plant Sci 14:1276424. https://doi.org/10.3389/fpls.2023.1276424
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Aust J Ecol 18:117–143. https://doi.org/10.1111/j.1442-9993.1993.tb00438.x
Clouse RM, Branstetter MG, Buenavente P, Crowley LM, Czekanski-Moir J, General DEM, Giribet G, Harvey MS, Janies DA, Mohagan AB (2017) First global molecular phylogeny and biogeographical analysis of two arachnid orders (Schizomida and Uropygi) supports a tropical Pangean origin and mid-Cretaceous diversification. J Biogeogr 44:2660–2672. https://doi.org/10.1111/jbi.13076
DANE – Departamento Nacional de Estadística (2005) Atlas estadístico de Colombia. http://sige.dane.gov.co/atlasestadistico. Accessed 4 June 2023
Etter A, van Wyngaarden W (2000) Patterns of landscape transformations in Colombia, whit emphasis in the Andean region. Ambio 29:432–439. https://doi.org/10.1579/0044-7447-29.7.432
Etter A, McAlpine C, Possingham H (2008) Historical analysis of the spatial and temporal drivers of landscape change in Colombia since 1500. Ann Am Assoc Geogr 98:2–23. https://doi.org/10.1080/00045600701733911
Etter A, Andrade A, Saavedra K, Amaya P, Cortés J, Arévalo P (2021) Ecosistemas colombianos: amenazas y riesgos. Editorial Pontificia Universidad Javeriana, Bogotá
Flórez DE, Cepeda J (2007) Esquizómidos. In: Amat-García G, Andrade CG, Amat-García E (eds) Libro Rojo de los Invertebrados terrestres de Colombia. Instituto de Ciencias Naturales (ICN), Universidad Nacional de Colombia, Conservación Internacional Colombia, Instituto Alexander von Humboldt, Ministerio de Ambiente, Vivienda y Crédito Territorial, Bogotá, pp 68–71
Giribet G, Moreno-González JA (2021) Notes on brooding in the arachnid order Schizomida. J Arachnol 49:410–414. https://doi.org/10.1636/JoA-S-20-091
Hammer O, Harper D, Ryan PD (2001) PAST: Paleontological Statistics software package for education and data analysis. Palaentol Electron 4:1–9
Harvey MS (2002) Short-range endemism amongst the Australian fauna: some examples from non-marine environments. Invertebr Syst 16:555–570. https://doi.org/10.1071/IS02009
Harvey MS (2003) Catalogue of the smaller arachnid orders of the world: Amblypygi, Uropygi, Schizomida, Palpigradi, Ricinulei and Solifugae. CSIRO Publishing, Melbourne
Humphreys WF, Adams M, Vine B (1989) The biology of Schizomus vinei (Chelicerata: Schizomida) in the caves of Cape Range, Western Australia. J Zool 217:177–201. https://doi.org/10.1111/j.1469-7998.1989.tb02481.x
Hurtado-M AB, Echeverry-Galvis MÁ, Salgado-Negret B, Muñoz JC, Posada JM, Norden N (2021) Little trace of floristic homogenization in peri-urban Andean secondary forests despite high anthropogenic transformation. J Ecol 109:1468–1478. https://doi.org/10.1111/1365-2745.13570
JASP TEAM 2023. JASP (Version 0.18.1.0). https://jasp-stats.org/. Accessed 19 June 2023.
Kallal RJ, de Miranda GS, Garcia EL, Wood H (2022) Patterns in schizomid flagellum shape from elliptical Fourier analysis. Sci Rep 12:3896. https://doi.org/10.1038/s41598-022-07823-y
Kraus O (1957) Schizomidae aus Kolumbien (Arach., Pedipalpi - Schizopeltidia). Senckenberg Biol 38:245–250
Kraus O, Beck L (1967) Taxonomie und Biologie von Trithyreus brasiliensis n. sp (Arach.: Pedipalpi: Schizopeltidia). Senckenberg Biol 48:401–405
Legendre P, Legendre L (1998) Numerical Ecology, 2nd English. Elsevier, Amsterdam
Magnussen IDF, Müller SP, Hammel JU, Kotthoff U, Harms D (2022) Diversity of schizomids (Arachnida: Schizomida) revealed by new fossil genera and species from mid-Cretaceous Burmese amber with implications for a Gondwanan origin of the Burma Terrane. Zool J Linn Soc 196:792–844. https://doi.org/10.1093/zoolinnean/zlac034
Monjaraz-Ruedas R, Francke OF, Cruz-López JA, Santibáñez-López CE (2016) Annuli and setal patterns in the flagellum of female micro-whipscorpions (Arachnida: Schizomida): hypotheses of homology across an order. Zool Anz 263:118–134. https://doi.org/10.1016/j.jcz.2016.05.003
Oliveira MPA, Ferreira RL (2014) Aspects of the behavior and activity rhythms of Rowlandius potiguar (Schizomida: Hubbardiidae). PLoS ONE 9:e91913. https://doi.org/10.1371/journal.pone.0091913
Reddell JR, Cokendolpher JC (1995) Catalogue, bibliography, and generic revision of the order Schizomida (Arachnida). Texas Memorial Mus Speleol Monogr 4:1–170
Sturm H (1958) Indirekte Spermatophorenübertragung bei dem Geisselskorpion Trithyreus sturmi Kraus (Schizomidae, Pedipalpi). Naturwissenschaften 45:142–143
Sturm H (1973) Zur Ethologie von Trithyreus sturmi Kraus (Arachnida, Pedipalpi, Schizopeltidia). Z Tierpsychol 33:113–140
Wild J, Kopecký M, Macek M, Šanda M, Jankovec J, Haase T (2019) Climate at ecologically relevant scales: a new temperature and soil moisture logger for long-term microclimate measurement. Agric for Meteorol 268:40–47. https://doi.org/10.1016/j.agrformet.2018.12.018
World Schizomida Catalog (2022) World Arachnid Catalog. In: Natural History Museum Bern. https://wac.nmbe.ch. Accessed 18 March 2024
Acknowledgements
This study was conducted as part of the Small Grant project “Dinámicas de regeneración y descomposición de hojarasca en un gradiente sucesional de bosque Altoandino”, funded by Universidad del Rosario. The establishment and monitoring of permanent plots were made possible through the financial support of Universidad del Rosario, Pontificia Universidad Javeriana, and Minciencias, under the leadership of Natalia Norden and Juan Posada. We express our gratitude to Brayan Polania-Camacho for his valuable collaboration during field trips and lab assistance. Special thanks to Laura Garzón-Salamanca for her contribution to the preparation of the map in Fig. 1. We are in debt to the owners of the private areas where we conducted this research for their generosity and hospitality. Lastly, we extend our thanks to the three reviewers and the editor for their insightful comments, which have significantly enhanced the quality of this paper.
Funding
Open Access funding provided by Colombia Consortium.
Author information
Authors and Affiliations
Contributions
DCF conceived the study, collected and analyzed the data, wrote the paper, prepared the figures, reviewed and edited drafts of the manuscript. CCA collected data, wrote the paper, reviewed and edited drafts of the manuscript. JAM identified the schizomids, wrote the paper, reviewed and edited drafts of the manuscript. JMP wrote the paper, reviewed and edited drafts of the manuscript, and acquired funding.
Corresponding author
Ethics declarations
Competing interests
The authors declare no potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10841_2024_565_MOESM1_ESM.tif
Fig. S1 Spearman correlations between 14 variables from the permanent plots. AGB = aboveground biomass, LAI = Leaf Area Index. The Spearman correlation rank (rho) is represented in each square, where red colors indicate negative relationships, while blue colors depict positive relationships. Significant relationships are flagged with *(P < 0.05), **(P < 0.01), ***(P < 0.001) (TIF 1303 KB)
10841_2024_565_MOESM2_ESM.tif
Fig. S2 Loadings of the eight variables related to the environmental and structural forest characteristics in the (a) PC1 and (2) PC2 (TIF 571 KB)
10841_2024_565_MOESM3_ESM.tif
Fig. S3 Sheppard plot showing the obtained vs observed (target) ranks that indicates the quality of the NMDS. Note that points are not strongly deviated from the straight ascending line 1:1, suggesting a good representation of the analyzed distances between objects without significant distortion (TIF 259 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Castillo-Figueroa, D., Castillo-Avila, C., Moreno-González, J.A. et al. Habitat of two threatened short-tailed whip-scorpions (Arachnida: Schizomida) in the tropical Andes of Northern South America. J Insect Conserv (2024). https://doi.org/10.1007/s10841-024-00565-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10841-024-00565-4