Abstract
Due to particular vulnerabilities and environmental constraints, Alpine faunas are exposed to significant threats from climate change. However, baseline diversity and distribution data to monitor the trends of key arthropod groups are often scarce. Ants are highly diversified and key ecological actors across terrestrial ecosystems, including mountain ranges. We investigated ant diversity and distribution in the Southern European Alps to provide detailed data over wide elevational gradients and make a first assessment of potential vulnerabilities in the face of global warming. We detected 40 species from 700 to over 2600 m asl, with progressively less diversity corresponding to higher elevations and lower temperatures. Maximum temperature was weakly related to ant diversity as compared to mean and minimum temperature. In shaping ant diversity, the highest elevation species had wide elevation ranges, consistent with Rapoport’s rule. We documented a fauna characterized by cold-adapted genera, species with wide geographic distributions and presumably high dispersal capabilities, no dietary specializations, and a high frequency of social parasitism. Concerning Bergmann’s rule, average ant species size was not larger in the coldest environments. Red wood ants, characterized by much more populous colonies as compared to the other ant species, had the highest number of ant individuals to be found at intermediate elevations and in woodland habitats. On the other hand, grasslands and shrublands proved to be the most species-rich habitats. Our data lay the groundwork for further investigation on elevational shifts and provide context for the discussion of key aspects of the management and conservation of European alpine ants.
Implications for insect conservation
The cold climate of the European Alps has so far protected its ant fauna from the incursion of alien competitors as compared to what is observed in the Mediterranean region. The scarcity of species with restricted distribution ranges or high-elevation specialists, as well as diet specialists, may grant ants of the European Alps more resilience to climate change as compared to other Alpine arthropod groups. On the other hand, many species are highly interconnected by social parasitism relationships that may be vulnerable to ecological cascade effects. While forest habitats host red wood ants, which are often protected for their important ecological role, the importance of grassland must not be overlooked as they support the highest ant species richness.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the context of climate change and insect decline (see Harvey et al. 2022), the alpine fauna may be especially vulnerable compared to those of other environments, having a higher proportion of less resilient specialist species, facing higher biogeographic isolation, and witnessing a faster pace of modifications (Hochkirch 2016; Wagner et al. 2021; Lencioni and Gobbi 2021). The ability of insect species to modify their distribution range to track the geographic shifts of tolerable climates is crucial to determine their chances to survive climatic changes: it depends on their natural history traits and on their ability to adapt their key traits quickly enough to the pace of the environmental modifications (Kellermann and van Heerwaarden 2019; Poloni et al. 2022). This can be especially difficult for alpine species that already live close to their physiological limits (Dahlhoff et al. 2019; Yadav et al. 2021). Upward elevational shifting is often the only possible response of alpine species facing increasing temperatures, yet it may cost a considerable reduction and fragmentation of the distribution range, and consequently of the population size; at the same time, species that already live on mountain peaks do not have this vital opportunity (Hodkinson 2005; Wilson et al. 2007; Shah et al. 2020; Halsch et al. 2021; Vitasse et al. 2021). Under current predictions, one third to more than a half of alpine and subalpine species may lose over 80% of suitable habitat by 2070 to 2100 (Engler et al. 2011), in addition, local extinctions of some taxa have been already documented (Panza and Gobbi 2022). However, baseline data on distribution and diversity in the European Alps, essential to monitor these trends, are still missing for several arthropod groups (Sommaggio et al. 2022).
Ants (Hymenoptera: Formicidae) are widespread and diverse across most terrestrial habitats of the globe often representing a remarkably high biomass, have a highly impactful ecological role through a multitude of relationships with animals, plants, fungi, and microbes, and can be used as ecological indicators (Hölldobler and Wilson 1990; Lach et al. 2010; Parker and Kronauer 2021; Schultheiss et al. 2022). Excluding the Mediterranean regions, the European fauna is very well resolved taxonomically, which represents an ideal context for ecological studies (Seifert 2018). Still, much of the existing ecological data have been produced under an outdated taxonomic framework (Seifert 2017). The European Alps are a key area for conservation biology due to multiple biogeographic aspects and its biota is threatened by rapid climatic alteration (Theurillat and Guisan 2001; Schwager and Berg 2019; Menchetti et al. 2021). Besides historic records, recent assessments on the European Alps ant fauna focus only on specific groups or deal with a narrow range of the elevational gradient (Glaser et al. 2012; Spotti et al. 2015; Krapf et al. 2018; Wagner 2020; Guariento and Fiedler 2021). Moreover, while there are literature data on the elevational range of most species, these are often based on decades-old studies (Seifert 2018). More generally, the role of climate change on the Alpine ant fauna has never been examined and no conservation priorities have been set.
As part of a wider long-term monitoring effort on the arthropod fauna of the Italian National Parks funded by the Italian Ministry for the Environment, we carried out a systematic investigation of ant diversity and distribution along elevational transects, aiming (i) to provide an updated reference on species elevational limits, (ii) to define the main diversity patterns and relationships with abiotic variables, as well as (iii) laying the groundwork for future conservation assessments by examining possible vulnerabilities to the ongoing climate change.
Materials and methods
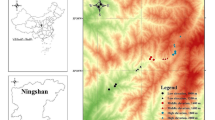
Sampling was conducted in two Italian National Parks located in the Southern Alps, the Dolomiti Bellunesi National Park (DBNP) and the Stelvio National Park (SNP) during the years 2018–2019 (Fig. 1). Elevational transects consisted of a variable number of sampling stations at different elevations, each represented by a group of five pitfall traps. The traps were built with plastic glasses of c. 6 cm in diameter and c. 7 cm in height, buried in the ground, filled with c. 150 ml cc of a preserving mixture of white vinegar, sodium chloride, and a drop of detergent as a surfactant, as described by Gobbi (2020). Traps were replaced every 16–18 days from May/June to September/October, resulting in 5 to 10 sampling sessions per year (a shorter sampling season and number of sessions per year correspond to higher elevations where the snow coverage lasts longer). Sampling covered elevations from 780 to 2130 m asl in the DBNP (2 elevational transects, 11 sampling stations, 55 traps) and from 1400 to 2690 m asl in the SNP (6 elevational transects, 30 sampling stations, 150 traps), for a total of 205 traps (Fig. 1). In both Parks, traps of the same sampling station were normally placed in the same habitat type, while different sampling stations covered the widest variety habitat types and were placed at an elevational difference of about 150–200 m from the next ones within the same transect. For each trap, we recorded the habitat type as well as the temperature throughout the sampling season using data loggers (Thermochrom i-Button), placed at the center of each sampling station. Habitats were grouped in three macro-categories for the analyses: woodlands (Fagus sylvatica, Larix decidua, Pinus sylvestris, and Picea abies forests; 52 traps from 17 sampling stations), grassland/shrublands (grasslands, shrublands, wetlands; 145 traps from 34 sampling stations), and scree slopes (10 traps from 3 sampling stations). The exposure duration of each trap, accounting for the lost session due to malfunctioning or lost samples, is described in the Supplementary table S1.
Sampling effort in the Dolomiti Bellunesi National Park (DBNP) and Stelvio National Park (SNP), in the Italian Alps. Each point in the map indicates a sampling station (11 sampling stations in the DBNP, 30 in the SNP), while the number of traps used at each elevational range is illustrated in the barplot. Map image from Stadia Maps and Stamen Design, modified
Ants were identified under a Zeiss Stemi 508 stereoscopic microscope, taking morphometric measurements with the aid of an Axiocam Erc 5s camera and the Zeiss Zen Core Software. Identification was based on the taxonomic keys provided by Radchenko and Elmes (2010), Wagner et al. (2017), and Seifert (2018). For what concerning the Tetramorium caespitum cryptic complex, a safe morphological identification should be based on quantitative morphological data from at least three workers belonging to the same colony, which cannot be ensured with pitfall trapping (Wagner et al. 2017). Therefore, we made an approximation suited to the purposes of this study, applying the key by Wagner et al. (2017) to up to 3 workers per trap.
Statistical analyses were based on ant workers only, thus excluding dispersing sexuals that may occur far from the suitable habitats of their species, except for queens of workerless social parasites collected alongside workers of their host (Schifani et al. 2021). Before all analyses, the number of workers per traps was divided by the number of days the traps were active in order to standardize the values based on the sampling effort. Simpson’s Index (1-D) values were computed for each trap to have a measure of diversity in addition to genus and species richness (Simpson 1949). Analyses on the number of individuals were conducted after logarithmically transforming their numbers to remove outliers that may have resulted from traps accidentally placed too close to ant nests. Species accumulation curves were computed using the function specaccum from the R package vegan (Oksanen et al. 2022) to compare the exploration of the eight transects.
Differences in genus and species richness, Simpson’s Index scores, and number of individuals between the three main habitat types (grasslands/shrubland, woodland, and scree) were assessed using mixed-effect models in which the Park was set as a random factor to account for the differences between distinct Alpine regions. Tukey’s post hoc tests using Bonferroni’s correction were used for pairwise comparisons.
We investigated whether species distribution followed Bergmann’s rule at the assemblage level (species of colder environments having a larger size, see Bergmann 1847) with a mixed-effect model with polynomial terms using the lmer function from the lme4 R package (Bates et al. 2015). We set the sampling station as the random factor while studying the relationship between mean temperature and the average cephalic size (the most used as a proxy for body size) among the ant species found in the traps (using community-weighted means, i.e. based on the occurrence and not the abundance of each species per trap), assigning to each species the corresponding mean value of cephalic size based on literature data (Seifert 2017, 2018). Cephalic size was calculated as the arithmetic mean between cephalic width and cephalic length (Seifert 2018).
Pearson’s correlation tests were used to verify whether species distribution abided to the Rapoport’s rule (species found at higher elevations having a smaller elevational range Stevens 1992): We correlated the maximum elevational limit with the difference between the maximum and minimum elevational ranges that we recorded for each species.
Finally, using the lmer function from the lme4 R package (Bates et al. 2015), mixed-effect models with polynomial terms were used to assess potential non-linear relationships between diversity measures (number of species, number of genera, Simpson’s Diversity Index, and number of individuals) and environmental variables (elevation, minimum, mean, and maximum temperature—using a model for each variable), while setting the sampling station as a random factor. This design was preferred to the use of a nested design with transect set as a nested factor within park, as well as a design with park (instead of the sampling station) as the sole random factor. The former resulted in similar AIC and BIC values while leading to singularity and convergence issues, while the latter resulted in significantly higher AIC and BIC values. A correlation plot illustrating the relationship between the four environmental variables (elevation, minimum, mean, and maximum temperature) is provided in the Supplementary Figure S2.
All statistical analyses were carried out using the software R v4.2.0 and RStudio-2022.02.2-485 (R Core Team 2022).
Results
A full dataset illustrating for each trap the number of ant specimens collected and their identity, as well as the environmental variables recorded, is provided in Supplementary Table S1. Species accumulation curves suggested that a satisfactory coverage was reached in all transects except for the transect MG of the DBNP, which did not fully reach a proper plateau (Fig. 2).
A total of 262,693 worker ant specimens were processed and identified during this study. They belonged to 40 species, 12 genera, and 3 subfamilies (Dolichoderinae, Formicinae, and Myrmicinae). Of these, the only finding of Formicoxenus nitidulus consisted of one winged queen which was excluded from the analysis. The most species-rich genera were Formica and Myrmica, both with 11 species, followed by Lasius, with 6 species. The most abundant species were Formica aquilonia (158,513 specimens) and Formica lugubris (75,698 specimens), while the most frequent were F. lugubris (found in 68 traps) and Tetramorium. alpestre (found in 42 traps). The species occurring at the highest elevations were Formica lemani, Myrmica lobulicornis, and T. alpestre, while Formica gagates, Plagiolepis pygmaea, and Lasius mixtus occurred only at the lowest elevations (Fig. 3).
Elevational distribution of the ant species found in the traps along our sampling transects, excluding dispersing sexuals from the analysis (Formicoxenus nitidulus is excluded since only a single dispersing queen was collected). Species are listed in an increasing order based on their upper elevational range
The highest diversity of species was found at intermediate elevations, between 1400 and 1800 m asl (Fig. 4). From 1600 to 2200 m asl most specimens belonged to mound-building Formica species (F. exsecta, F. truncorum, and the F. rufa group, including F. aquilonia, F. lugubris, and F. rufa), while at higher elevations other Formica species were found (mainly F. cunicularia, F. lemani or F. rufibarbis, Fig. 4). The highest elevation was reached by F. lemani, found at 2631 m asl. At the species-level, the prevalent genera were Formica and Myrmica across all elevations, while a significant portion of Lasius existed until 1800 m and Tetramorium was one of the three genera recorded above 2400 m (Fig. 4). On the other hand, at the individual level, after 1600 m, more than 95% of the captured specimens belonged to Formica, with mound-building species being replaced by F. lemani above 2400 m (Fig. 4).
Concerning the comparison among habitat types, statistically significant differences were found for the number of species (F(2202) = 8.62, p < 0.001), genera (F(2201) = 7.97, p < 0.001), and individuals (F(2202) = 21.63, p < 0.001), as well as for the Simpson’s Index values (F(2201) = 5.93, p < 0.001) (Fig. 5). Pairwise comparisons of the number of species revealed significant differences between grassland/shrubland, having the highest species richness values, and both scree (p = 0.002) and woodlands (p = 0.040), but not between woodlands and scree (p = 0.101). For the number of genera, scree had the lowest values and differed significantly from both grasslands/shrublands (p = 0.003) and woodlands (p = 0.004), while grasslands/shrublands was not different from woodland (p = 0.573). Woodlands and scree were again not significantly different for Simpson’s Index values, having the lowest values (p = 0.175), while grasslands/shrublands differed from scree (p = 0.009) but not from woodlands (p = 0.093). Finally, the number of individuals showed a discordant trend, with all habitats significantly different from one another (p < 0.001 for all comparisons), woodlands having the highest numbers, scree the lowest, and grasslands/shrublands holding an intermediate position (Fig. 5).
Differences in abundance and diversity of ants among habitat macro-categories based on our sampling transects (n traps: scree = 10; woodlands = 52; grasslands/shrublands = 143). Habitats that are significantly different based on pairwise comparisons (Tukey’s post hoc tests) are marked with different letters
Concerning Bergmann’s rule, a significant relationship was found between minimum temperature increase and the average cephalic size of ant assemblages (F(238) = 3.79, p < 0.031, R2 = 0.45; estimate1: 389, p = 0.59; estimate2: − 1765, p = 0.02). Ants associated with intermediate temperature ranges, instead of coldest temperatures, showing the larger size (Fig. 6). For Rapoport’s rule, we found a strong positive correlation between species’ elevational range and their maximum elevational limit (t(37) = 6.30, p < 0.001, R = 0.72) (Fig. 6).
Relationship between ant size (with cephalic size as a proxy) of the collected ants and minimum temperature recorded at the corresponding sampling station (on the left), and relationship between the maximum elevational limit and the elevational range of each species (on the right). According to the Bergmann’s rule, size of organisms should increase at lower temperatures, while Rapoport’s rule predicts that species found at higher elevations are characterized by a larger elevational range
Significant relationships were found between each diversity measure (number of species, number of genera, Simpson’s Diversity Index, and number of individuals) and environmental variable (elevation, minimum, mean, and maximum temperature) (0.034 < p < 0.001), except for the number of individuals and maximum temperature (p = 0.117) (see Supplementary Table S3, Fig. 7). The comparison of AIC and BIC values between models that included different environmental variables revealed the lowest scores for the effect of mean temperature except for the models on the number of individuals, for which the lowest AIC and BIC scores corresponded to the minimum temperature. The highest AIC and BIC scores always corresponded to the models with the maximum temperature (Supplementary Table S3). Ant diversity (number of species, number of genera, Simpson’s diversity index) and abundance (number of individuals) mostly decreased as expected and increased with temperature, but in most cases an opposite trend was observed at the lowest altitudes or highest temperatures recorded, resulting in a non-linear relationship (Fig. 7).
Discussion
Our study provides a detailed reference dataset as an essential tool to monitor ant elevational distribution in the European Alps and observe future shifts in response to climatic change, also laying the ground to discuss conservation priorities and better-include ants in management plans. Overall, the species diversity we found represents approximately 21% of the central and Northern European fauna and 15% of the Italian fauna (Seifert 2018; Schifani 2022).
The European Alps ant fauna appears characterized by a remarkable proportion of the European diversity of the cold-adapted genera Formica and Myrmica (Seifert 2018), and a very significant representation of social parasitism (Buschinger 2009). All socially parasitic strategies were represented: dulosis (F. sanguinea); temporary social parasitism (F. aquilonia, F. exsecta, F. sanguinea, F. lugubris, F. rufa, F. truncorum, L. mixtus, L. umbratus) and hyperparasitism (L. fuliginosus); inquilinism (F. nitidulus, M. microrubra, M. myrmicoxena) (Buschinger 2009; Seifert 2018). Inquiline social parasite species are unlikely to be captured with pitfall trapping, and parasite Temnothorax, Tetramorium or Strongylognathus may have been present but were not captured. At the same time, compared to what is observed in more thermophilous regions of Europe (e.g., Castracani et al. 2010; Bazzato et al. 2022), species diversity per site was lower, and there were no diet specialists (such as granivorous species or specialized predators) (Seifert 2018). Myrmica myrmicoxena, listed as Vulnerable in the IUCN 2.3 Red List together with F. nitidulus, was the only Alpine endemic species collected (Social Insects Specialist Group 1996; Schifani et al. 2021), while all other ant species are characterized by wide distribution ranges, mostly covering continental Europe and in many cases extending to other high-latitude Palearctic regions (Seifert 2018; Schär et al. 2018; Wang et al. 2022). We detected a complex relationship between ant size and temperature, not clearly following Bergmann’s rule. In areas with the lowest temperatures many small sized genera did not occur (e.g. Leptothorax, Plagiolepis, Tapinoma, Temnothorax), but also the largest sized taxa were absent (Camponotus spp., mound-building Formica species), while ant size was larger at intermediate temperature range, a pattern largely driven by mound-building Formica species. Previous studies on different scales found mixed support for the Bergmann’s rule in ants, with general trends at least less pronounced than in other animal groups (Kaspari and Vargo 1995; Geragthy et al. 2007), and in some cases the observed trend being the opposite to that described by the Bergmann’s rule (Brassard et al. 2020). Furthermore, it may still be possible that intraspecific patterns conform to Bergmann’s rule better than interspecific ones or that the rule gains support only by sampling a much wider thermal gradient.
The abundant presence of the iconic mound-building Formica species, mostly belonging to the F. rufa group (red wood ants), had a major role in the data we collected. Since their normal colony size is several times larger than in most other alpine ant species (Seifert 2018), their frequent presence in woodlands at intermediate elevations determined the highest absolute number of ants to be found in these habitats. These ants are behaviorally dominant to other ants (Lach et al. 2010), and are considered ecological keystones acting as important forest predators and trophobionts, hosting a variety of associated myrmecophilic organisms, but at the same time significantly lowering the diversity and abundance of many other arthropods in their territory, including other ants, as a consequence of competition or territorialism (Ślipiński et al. 2014; Stockan and Robinson 2016; Trigos-Peral et al. 2016; Seifert 2017; 2018; Parmentier and Claus 2019; Castellucci et al. 2022). Red wood ants have historically attracted higher conservation attention than any other European ant, and efforts to monitor their presence are frequent, yet no specific concerns for the alpine populations have been identified so far (Antonova and Marinov 2021; Sorvari 2022; Van Buggenum 2022; Balzani et al. 2022).
Ant abundance and diversity expectedly decreased with elevation and increased with temperature (Reymond et al. 2013). The relationship tended to be stronger with mean temperature and always weaker with the maximum temperature. Exploring the role of thermal limits in ant biogeographic patterns is an important and rapidly developing field with obvious relevance to climate change scenarios (Nascimento et al. 2022; Willot et al. 2022; 2023; Araujo et al. 2023). Species adapted to tolerate the more extreme elevations were characterized by larger elevational ranges, in accordance with the Rapoport’s rule (Stevens 1992). In this sense, alpine ants appear to be less susceptible to the direct effects of temperature increases compared to organisms of other groups (Gobbi 2020; Pallarés et al. 2020; Poloni et al. 2022). At the same time, the expansion of dominant forest ants favored by a general increase of forested areas may have significant impacts on the diversity of both ants and other arthropod groups (Bebi et al. 2017). In general, ant communities became very species-poor above the tree line (> 2200 m asl), as only three species (F. lemani, M. lobulicornis, T. alpestre) were found in the highest grasslands and screes, where energy nutrients may be particularly limiting (Guariento et al. 2021)—each of them occurring across an elevational range of at least 1000 m. Maximum elevational ranges recovered in this study mostly corresponded to those reported in the literature, with no indication of rapid upward range shifts taking place as in other groups (Brambilla and Gobbi 2014; Gilgado et al. 2022; Panza and Gobbi 2022). Open habitats like grasslands and shrublands were those that hosted the highest species diversity. This is likely a combined result of higher topsoil temperatures due to reduced shade, and reduced presence of territorial and behaviorally dominant ant species (Seifert 2017). Therefore, preserving open habitats appears to be fundamental for the conservation of alpine ants as it happens with other arthropod groups (Bussan 2022; Gobbi et al. 2015, 2022; Walcher et al. 2019; Schwarz and Fartmann 2021). Dispersal ability may play a crucial role in future scenarios for alpine organisms but very little is known in this regard for most European ant species. However, all the species we detected are capable of flight dispersal (Seifert 2018). Interestingly, we once found a L. fuliginosus queen at 2400 m asl, several hundred meters above the highest elevation recorded for the colonies of this parasitic species or its hosts: upward winds are likely to favor occasional dispersal of alates above the suitable elevational limit of their species.
In conclusion, the ant fauna we investigated appears to be well-characterized by the richness of cold-adapted taxa and the peculiar prominent role of red wood ants near woodlands. The scarcity of endemics or species with narrow distribution ranges, including at the highest elevations, as well as the absence of diet specialists and the presumably good dispersal capabilities of the occurring species, may perhaps grant Alpine ants some more resilience to climate-driven environmental changes compared to other arthropod groups. The cold climate conditions also prevent the establishment of most invasive ant species that significantly threaten ant diversity elsewhere in Europe (Menchetti et al. 2023; Schifani 2019). On the other hand, strong interconnections between species in the form of widespread social parasitism may represent a special weakness to potential ecological cascade effects. Further efforts are required to monitor elevational shifts over time and genetic diversity along elevational gradients, as well as the ecological relationships between ants and other key arthropod groups in the Alpine environment.
Change history
13 April 2024
A Correction to this paper has been published: https://doi.org/10.1007/s10841-024-00573-4
References
Antonova V, Marinov MP (2021) Red wood ants in Bulgaria: distribution and density related to habitat characteristics. J Hymenopt Res 85:135–159. https://doi.org/10.3897/jhr.85.61431
Araujo NDS, Perez R, Willot Q, Defrance M, Aron S (2023) Facing lethal temperatures: heat-shock response in desert and temperate ants. Ecol Evol 13:e10438. https://doi.org/10.1002/ece3.10438
Balzani P, Dekoninck W, Feldhaar H, Freitag A, Frizzi F, Frouz J, Masoni A, Robinson E, Sorvari J, Santini G (2022) Challenges and a call to action for protecting European red wood ants. Conserv Biol 36:e13959. https://doi.org/10.1111/cobi.13959
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting Linear mixed-effects models using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Bazzato E, Lallai E, Caria M, Schifani E, Cillo D, Ancona C, Alamanni F, Pantini P, Maccherini S, Bacaro S, Marignani M (2022) Land-use intensification reduces multi-taxa diversity patterns of small woodlots outside forests in a Mediterranean area. Agric Ecosyst Environ 340:108149. https://doi.org/10.1016/j.agee.2022.108149
Bebi P, Seidl R, Motta R, Fuhr M, Firm D, Krumm F, Conedera M, Ginzler C, Wohlgemuth T, Kulakowski D (2017) Changes of forest cover and disturbance regimes in the mountain forests of the Alps. For Ecol Manag 388:43–56. https://doi.org/10.1016/j.foreco.2016.10.028
Bergmann C (1847) Über die Verhältnisse Der Wärmeökonomie Der Thiere zu Ihrer Grösse. Göttinger Studien 3:595–708
Brambilla M, Gobbi M (2014) A century of chasing the ice: delayed colonisation of ice-free sites by ground beetles along glacier forelands in the Alps. Ecography 37:33–42. https://doi.org/10.1111/j.1600-0587.2013.00263.x
Brassard F, Francoeur A, Lessard JP (2020) Temperature drives caste-specific morphological clines in ants. J Anim Ecol 89:2517–2530. https://doi.org/10.1111/1365-2656.13330
Van Buggenum HJ (2022) Presence after three decades of red wood ants (Formica rufa group; Hymenoptera: Formicidae) in forests in an agricultural landscape. Eur J Entomol 119:85–91. https://doi.org/10.14411/eje.2022.009
Buschinger A (2009) Social parasitism among ants: a review (Hymenoptera: Formicidae). Myrmecol News 12:219–235
Bussan SK (2022) Can cattle grazing benefit grassland butterflies? J Insect Conserv 26:359–374. https://doi.org/10.1007/s10841-022-00373-8
Castellucci F, Schifani E, Luchetti A, Scharff N (2022) New association between red wood ant species (Formica rufa group) and the myrmecophilic spiders Mastigusa arietina and Thyreosthenius biovatus. Bull Insectol 75:231–238
Castracani C, Grasso DA, Fanfani A, Mori A (2010) The ant fauna of Castelporziano Presidential Reserve (Rome, Italy) as a model for the analysis of ant community structure in relation to environmental variation in Mediterranean ecosystems. J Insect Conserv 14:585–594. https://doi.org/10.1007/s10841-010-9285-3
Dahlhoff EP, Dahlhoff VC, Grainger CA, Zavala NA, Otepola-Bello D, Sargent BA, Roberts KT, Heidl SJ, Smiley JT, Rank NE (2019) Getting chased up the mountain: high elevation may limit performance and fitness characters in a montane insect. Funct Ecol 33:809–818. https://doi.org/10.1111/1365-2435.13286
Engler R, Randin CF, Thuiller W, Dullinger S, Zimmermann NE, Araújo MB, Pearman PB, Lay GW, Piedallu C, Albert CH, Choler P, Coldea G, De Lamo X, Dirnböck T, Gégout J-C, Gómez-García D, Grytnes J-A, Heegard E, Høistad F, Nogués-Bravo D, Normand S, Puşcaş M, Sebastià M-T, Stanisci A, Theurillat J-P, Trivedi MR, Vittoz P, Guisan A (2011) 21st Century climate change threatens mountain flora unequally across Europe. Glob Change Biol 17:2330–2341. https://doi.org/10.1111/j.1365-2486.2010.02393.x
Geragthy MJ, Dunn RR, Sanders NJ (2007) Body size, colony size, and range size in ants (Hymenoptera: Formicidae): are patterns along elevational and latitudinal gradients consistent with Bergmann’s rule? Myrmecol News 10:51–58
Gilgado JD, Rusterholz HP, Baur B (2022) Millipedes step up: species extend their upper elevational limit in the Alps in response to climate warming. Insect Conserv Diver 15:61–72. https://doi.org/10.1111/icad.12535
Glaser F, Freitag A, Martz H (2012) Ants (Hymenoptera: Formicidae) in the Münstertal (val Müstair): a hot spot of regional species richness between Italy and Switzerland. Gredleriana 12:273–284
Gobbi M (2020) Global warning: challenges, threats and opportunities for ground beetles (Coleoptera: Carabidae) in high altitude habitats. Acta Zool Acad Sci Hung 66:5–20. https://doi.org/10.17109/AZH.66.Suppl.5.2020
Gobbi M, Fontaneto D, Bragalanti N, Pedrotti L, Lencioni V (2015) Carabid beetle (Coleoptera: Carabidae) richness and functional traits in relation to differently managed grasslands in the Alps. Ann Soc Entomol Fr 51:52–59. https://doi.org/10.1080/00379271.2015.1060008
Gobbi M, Corlatti L, Caccianiga M, ter Braak CJF, Pedrotti L (2022) Hay meadows’ overriding effect shapes ground beetle functional diversity in mountainous landscapes. Ecosphere 13:e4193. https://doi.org/10.1002/ecs2.4193
Guariento E, Fiedler K (2021) Ant diversity and community composition in alpine tree line ecotones. Insects 12:219. https://doi.org/10.3390/insects12030219
Guariento E, Wanek W, Fiedler K (2021) Consistent shift in nutritional ecology of ants reveals trophic flexibility across alpine tree-line ecotones. Ecol Entomol 46:1082–1092. https://doi.org/10.1111/een.13052
Halsch CA, Shapiro AM, Fordyce JA, Nice CC, Thorne JH, Waetjen DP, Forister ML (2021) Insects and recent climate change. Proc Natl Acad Sci U S A 118:e2002543117. https://doi.org/10.1073/pnas.2002543117
Harvey JA, Tougeron K, Gols R, Heinen R, Abarca M, Abram PK, Basset Y, Berg M, Boggs C, Brodeur J, Cardoso P, de Boer JG, De Snoo GR, Deacon C, Dell JE, Desneux N, Dillon ME, Duffy GA, Dyer LA, Ellers J, Espíndola A, Fordyce J, Forister ML, Fukushima C, Gage MJG, García-Robledo C, Gely C, Gobbi M, Hallmann C, Hance T, Harte J, Hochkirch A, Hof C, Hoffmann AA, Kingsolver JG, Lamarre GPA, Laurance WF, Lavandero B, Leather SR, Lehmann P, Le Lann C, López-Uribe MM, Ma C-S, Ma G, Moiroux J, Monticelli L, Nice C, Ode PJ, Pincebourde S, Ripple WJ, Rowe M, Samways MJ, Sentis A, Shah AA, Stork N, Terblanche JS, Thakur MP, Thomas MB, Tylianakis JM, Van Baaren J, Van de Pol M, Van der Putten WH, Van Dyck H, Verberk WCEP, Wagner WL, Weisser WW, Wetzel WC, Woods HA, Wyckhuys KAG, Chown SL (2022) Scientists’ warning on climate change and insects. Ecol Monogr. https://doi.org/10.1002/ecm.1553
Hochkirch A (2016) The insect crisis we can’t ignore. Nature 539:141. https://doi.org/10.1038/539141a
Hodkinson ID (2005) Terrestrial insects along elevation gradients: species and community responses to altitude. Biol Rev 80:489–513. https://doi.org/10.1017/S1464793105006767
Hölldobler B, Wilson EO (1990) The ants. Harvard University Press, Cambridge
Kaspari M, Vargo EL (1995) Colony size as a buffer against seasonality: Bergmann’s rule in social insects. Am Nat 145:610–632. https://doi.org/10.1086/285758
Kellermann V, van Heerwaarden B (2019) Terrestrial insects and climate change: adaptive responses in key traits. Physiol Entomol 44:99–115. https://doi.org/10.1111/phen.12282
Krapf P, Russo L, Arthofer W, Möst M, Steiner FM, Schlick-Steiner BC (2018) An Alpine ant’s behavioural polymorphism: monogyny with and without internest aggression in Tetramorium Alpestre. Ethol Ecol Evol 30:220–234. https://doi.org/10.1080/03949370.2017.1343868
Lach L, Parr C, Abbott K (2010) Ant ecology. Oxford University Press, Oxford
Lencioni V, Gobbi M (2021) Monitoring and conservation of cryophilous biodiversity: Concerns when working with insect populations in vanishing glacial habitats. Insect Conservation and Diversity 14(6):723–729. https://doi.org/10.1111/icad.12522
Menchetti M, Talavera G, Cini A, Salvati V, Dincă V, Platania L, Bonelli S, Balletto E, Vila R, Dapporto L (2021) Two ways to be endemic. Alps and apennines are different functional refugia during climatic cycles. Mol Ecol 30:1297–1310. https://doi.org/10.1111/mec.15795
Menchetti M, Schifani E, Alicata A, Cardador L, Sbrega E, Toro-Delgado E, Vila R (2023) The invasive ant Solenopsis invicta is established in Europe. Current Biology 33(17):PR896-R897. https://doi.org/10.1016/j.cub.2023.07.036
Nascimento G, Câmara T, Arnan X (2022) Critical thermal limits in ants and their implications under climate change. Biological Reviews 97(4):1287–1305. https://doi.org/10.1111/brv.12843
Oksanen J, Simpson G, Blanchet F, Kindt R, Legendre P, Minchin P, O’Hara R, Solymos P, Stevens M, Szoecs E, Wagner H, Barbour M, Bedward M, Bolker B, Borcard D, Carvalho G, Chirico M, De Caceres M, Durand S, Evangelista H, FitzJohn R, Friendly M, Furneaux B, Hannigan G, Hill M, Lahti L, McGlinn D, Ouellette M, Ribeiro Cunha E, Smith T, Stier A, Ter Braak C, Weedon J (2022) Vegan: community Ecology Package. R package version 2.6-4.
Pallarés S, Millán A, Mirón JM, Velasco J, Sánchez-Fernández D, Botella‐Cruz M, Abellán P (2020) Assessing the capacity of endemic alpine water beetles to face climate change. Insect Conserv Diver 13:271–282. https://doi.org/10.1111/icad.12394
Panza R, Gobbi M (2022) Areal contraction, upward shift and habitat fragmentation in the cold-adapted ground beetle Nebria Germarii Heer, 1837 in the Brenta Dolomites. Italy Rend Fis Acc Lincei 33:923–931. https://doi.org/10.1007/s12210-022-01112-6
Parker J, Kronauer DJ (2021) How ants shape biodiversity. Curr Biol 31:R1208–R1214. https://doi.org/10.1016/j.cub.2021.08.015
Parmentier T, Claus R (2019) A dazzling number of beetles (Coleoptera) in a hibernating nest of red wood ants, Formica rufa Linnaeus (Hymenoptera: Formicidae). Coleopt Bull 73:761–764. https://doi.org/10.1649/0010-065X-73.3.761
Poloni R, Iannella M, Fusco G, Fattorini S (2022) Conservation biogeography of high-altitude longhorn beetles under climate change. Insect Conserv Diver 15:429–444. https://doi.org/10.1111/icad.12570
Radchenko A, Elmes GW (2010) Myrmica ants (Hymenoptera: Formicidae) of the old world. Natura optima dux Foundation, Warszawa
RStudio Team (2020). RStudio: Integrated Development for R. RStudio, PBC, Boston, MA. http://www.rstudio.com/
Reymond A, Purcell J, Cherix D, Guisan A, Pellissier L (2013) Functional diversity decreases with temperature in high elevation ant fauna. Ecol Entomol 38:364–373. https://doi.org/10.1111/een.12027
Schär S, Talavera G, Espadaler X, Rana JD, Andersen Andersen A, Cover SP, Vila R (2018) Do holarctic ant species exist? Trans-Beringian dispersal and homoplasy in the Formicidae. J Biogeogr 45:1917–1928. https://doi.org/10.1111/jbi.13380
Schifani E (2019) Exotic ants (Hymenoptera, Formicidae) invading Mediterranean Europe: a brief summary over about 200 years of documented introductions. Sociobiology 66:198–208. https://doi.org/10.13102/sociobiology.v66i2.4331
Schifani E (2022) The new checklist of the Italian Fauna: Formicidae. Biogeographia 37:ucl006. https://doi.org/10.21426/B637155803
Schifani E, Castracani C, Spotti FA, Giannetti D, Ghizzoni M, Gobbi M, Lencioni V, Pedrotti L, Grasso DA, Mori A (2021) Social parasite ants in the alps: a new site of the vulnerable Myrmica myrmicoxena and new uppermost elevational limit for M. Microrubra. Sociobiology 68:e7176. https://doi.org/10.13102/sociobiology.v68i4.7276
Schultheiss P, Nooten SS, Wang R, Wong MK, Brassard F, Guénard B (2022) The abundance, biomass, and distribution of ants on earth. PNAS. https://doi.org/10.1073/pnas.2201550119
Schwager P, Berg C (2019) Global warming threatens conservation status of alpine EU habitat types in the European Eastern Alps. Reg Environ Change 19:2411–2421. https://doi.org/10.1007/s10113-019-01554-z
Schwarz C, Fartmann T (2021) Conservation of a strongly declining butterfly species depends on traditionally managed grasslands. J Insect Conserv 25:255–271. https://doi.org/10.1007/s10841-020-00288-2
Seifert B (2017) The ecology of central European non-arboreal ants–37 years of a broad-spectrum analysis under permanent taxonomic control. Soil Org 89:1–69
Seifert B (2018) Ants of northern and central Europe. Lutra Verlags- und Vertriebsgesellschaft, Tauer
Shah AA, Dillon ME, Hotaling S, Woods HA (2020) High elevation insect communities face shifting ecological and evolutionary landscapes. Curr Opin Insect Sci 41:1–6. https://doi.org/10.1016/j.cois.2020.04.002
Simpson, E.H. (1949). Measurement of diversity. Nature, 163, 688.
Ślipiński P, Marko B, Rzeszowski K, Babik H, Czechowski W (2014) Lasius fuliginosus (Hymenoptera: Formicidae) shapes local ant assemblages. North-West J Zool 10:404–412
Social Insects Specialist Group (1996) Formicidae. In: 2006 IUCN Red List of Threatened Species. Available from: http://www.iucnredlist.org
Sommaggio D, Zanotelli L, Vettorazzo E, Burgio G, Fontana P (2022) Different distribution patterns of hoverflies (Diptera: Syrphidae) and bees (Hymenoptera: Anthophila) along elevational gradients in Dolomiti Bellunesi National Park (Italy). Insects 13:293. https://doi.org/10.3390/insects13030293
Sorvari J (2022) Biogeography and habitat preferences of red wood ants of the Formica rufa group (Hymenoptera: Formicidae) in Finland, based on citizen science data. Eur J Entomol 119:92–98. https://doi.org/10.14411/eje.2022.010
Spotti FA, Castracani C, Grasso DA, Mori A (2015) Daily activity patterns and food preferences in an alpine ant community. Etho Ecol Evol 27:306–324. https://doi.org/10.1080/03949370.2014.947634
Stevens GC (1992) The elevational gradient in altitudinal range: an extension of Rapoport’s latitudinal rule to altitude. Am Nat 140:893–911. https://doi.org/10.1086/285447
Stockan J, Robinson E (2016) Wood ant ecology and conservation. Cambridge University Press, Cambridge. https://doi.org/10.1017/CBO9781107261402
Theurillat JP, Guisan A (2001) Potential impact of climate change on vegetation in the European Alps: a review. Clim Change 50:77–109. https://doi.org/10.1023/A:1010632015572
Trigos-Peral G, Marko B, Babik H, Tăuşan I, Maák IE, Pálfi Z, Czechowski W (2016) Differential impact of two dominant Formica ant species (Hymenoptera, Formicidae) on subordinates in temperate Europe. J Hymenopt Res 50:97–116. https://doi.org/10.3897/JHR.50.8301
Vitasse Y, Ursenbacher S, Klein G, Bohnenstengel T, Chittaro Y, Delestrade A, Monnerat C, Rebetez M, Rixen C, Strebel N, Schmidt BR, Wipf S, Wohlgemuth T, Yoccoz NG, Lenoir J (2021) Phenological and elevational shifts of plants, animals and fungi under climate change in the European Alps. Biol Rev 96:1816–1835. https://doi.org/10.1111/brv.12727
Wagner HC (2020) The geographic distribution of ants (Hymenoptera: Formicidae) in Styria (Austria) with a focus on material housed in the Universalmuseum Joanneum. Joannea Zool 18:33–152
Wagner HC, Arthofer W, Seifert B, Muster C, Steiner FM, Schlick-Steiner BC (2017) Light at the end of the tunnel: integrative taxonomy delimits cryptic species in the Tetramorium caespitum complex (Hymenoptera: Formicidae). Myrmecol News 25:95–129
Wagner DL, Grames EM, Forister ML, Berenbaum MR, Stopak D (2021) Insect decline in the Anthropocene: death by a thousand cuts. Proc Natl Acad Sci U S A 118:e2023989118. https://doi.org/10.1073/pnas.2023989118
Walcher R, Hussain RI, Sachslehner L, Bohner A, Jernej I, Zaller JG, Arnberger A, Frank T (2019) Long-term abandonment of mountain meadows affects bumblebees, true bugs and grasshoppers: a case study in the Austrian Alps. Appl Ecol Environ Res 17:5887–5908. https://doi.org/10.15666/aeer/1703_58875908
Wang R, Kass JM, Galkowski C, Garcia F, Hamer MT, Radchenko A, Salata S, Schifani E, Yusupov ZM, Economo EP, Guénard B (2022) New distribution data and phylogenetic approach reveal bioregionalization of European ants. J Biogeogr 50:503–514. https://doi.org/10.1111/jbi.14546
Willot Q, Ørsted M, Damsgaard C, Overgaard J (2022) Thermal-death-time model as a tool to analyze heat tolerance, acclimation, and biogeography in ants. Myrmecol News 32:127–138. https://doi.org/10.25849/myrmecol.news_032:127
Willot Q, Ørsted M, Malte H, Overgaard J (2023) Cold comfort: metabolic rate and tolerance to low temperatures predict latitudinal distribution in ants. Proc R Soc B: Biol Sci. https://doi.org/10.1098/rspb.2023.0985
Wilson RJ, Gutierrez D, Gutierrez J, Monserrat VJ (2007) An elevational shift in butterfly species richness and composition accompanying recent climate change. Glob Change Biol 13:1873–1887. https://doi.org/10.1111/j.1365-2486.2007.01418.x
Yadav S, Stow AJ, Dudaniec RY (2021) Microgeographical adaptation corresponds to elevational distributions of congeneric montane grasshoppers. Mol Ecol 30:481–498. https://doi.org/10.1111/mec.15739
Acknowledgements
We thank the staff of the Dolomiti Bellunesi and the Stelvio National Park for the logistic support to the research. For the Stelvio National Park, Alessandro Gugiatti and Paolo Belotti supervised and managed the field survey of the epigean arthropod fauna. Teresa Boscolo and Michael Bernasconi (MUSE-Science Museum of Trento, Italy) sorted the Formicidae collected by pitfall traps, Alessandra Franceschini (MUSE-Science Museum of Trento, Italy) helped in collections management, and Valeria Lencioni (MUSE-Science Museum of Trento, Italy) helped in founding management. For the Dolomiti Bellunesi National Park, Giovanni Timossi carried out the fieldwork and sorted the Formicidae collected by pitfall traps. Moreover, we thank Myriam Di Capo and Giulia Fantoni for their assistance in sample organization during their internship at the University of Parma. Finally, we are grateful for two anonymous referees whose suggestions improved our manuscript.
Funding
Open access funding provided by Università degli Studi di Parma within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
Conceptualization: ES, CC, FAS; methodology: ES, MG, LP, EV; validation: CC, FAS, MG; formal analysis: ES; investigation: ES, LP, EV, MG; resources: CC, FAS, MG, LP, EV, DAG, AM; data curation: ES, LP, EV, CC, FAS; writing—original draft: ES; writing—review and editing: MG, FAS, CC, LP, EV, DAG, AM, ES; visualization: ES, CC, FAS; supervision: CC, AM, DAG; project administration: LP, MG, EV, AM, CC, FAS; funding acquisition: CC, LP, MG, EV, AM, DAG.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised due to the Abstract, legend of Figure 6 and Discussion section have been corrected.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schifani, E., Grasso, D.A., Gobbi, M. et al. Ant diversity along elevational gradients in the European Alps: insights for conservation under a changing climate. J Insect Conserv 28, 401–413 (2024). https://doi.org/10.1007/s10841-023-00546-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-023-00546-z