Abstract
Coppice forests are socio-ecological systems especially rich in biodiversity. They have been transformed into high forest and abandoned across large areas of Europe over the past 200 years. Coppice loss is likely an important driver of insect declines. It is currently unclear whether habitat quality or decreasing connectivity of the remaining fragments is more important for the survival of insect populations. We related the abundance of two coppice-associated butterflies of conservation concern, Satyrium ilicis and Melitaea athalia, to indicators of habitat quality and habitat connectivity. We estimated butterfly densities using Distance Sampling along a successional gradient (time since last cut: 1–9 years; N = 130 plots) across one of the largest remaining simple oak-birch coppice landscapes in Central Europe. Both species reached abundance peaks within four to six years after the last cut, declining rapidly in abundance with subsequent succession. We found no evidence that coupe size, coppice availability and patch (= coupe) connectivity were related to the density of the species. Besides stand age, the cover of larval foodplants explained predicted butterfly densities well. Only Satyrium ilicis benefitted from high Red Deer densities.
Implications for insect conservation: Our results suggest that habitat quality and sufficient availability of coppice of suitable age matters more than coupe size and fragmentation within a traditional managed coppice landscape. Coppice restoration aiming at the study species should ensure a shifting mosaic of successional habitat to provide a large availability of resprouting oak stools and bilberry vegetation that holds dense Melampyrum pratense stands.
Similar content being viewed by others
Introduction
Habitat loss through anthropogenic land-use change is the most important driver of biodiversity decline globally (Jaureguiberry et al. 2022). Habitat loss has large, consistently negative effects on biodiversity (Fahrig 2003). The effects of habitat fragmentation are less clear. Habitat fragmentation is often defined as the division of habitat into smaller and more isolated fragments, separated by a matrix of human-transformed land cover (Haddad et al. 2015). In a global meta-analysis of experimental studies, habitat fragmentation has been shown to reduce biodiversity by 13 to 75% (Haddad et al. 2015). But habitat fragmentation, once controlled for the loss of habitat amount (habitat fragmentation ‘per se’), can also result in biodiversity gains and positive population responses (Fahrig 2017). However, definitions and approaches to study habitat fragmentation vary across studies, and are therefore difficult to interpret and compare (Fischer and Lindenmayer 2007). There is considerable debate about the effects of habitat fragmentation on biodiversity (Fahrig 2017; Fletcher et al. 2018; Fahrig et al. 2019).
In applied conservation, e.g. the management of Protected Areas, it is often of interest to estimate effects of habitat fragmentation versus those of the loss of habitat amount, or habitat quality. Understanding whether total habitat amount, patch size or habitat quality contribute most to species richness and abundance allows conservation managers to develop appropriate strategies, assessing trade-offs between improving habitat quality and fragmentation of habitats. Habitat quality but also habitat availability at a landscape scale have repeatedly been shown to be more important drivers of occurrence, abundance and diversity than fragmentation, i.e. habitat isolation (Poniatowski et al. 2018; Merckx et al. 2019; Silva et al. 2022).
Habitat loss and fragmentation are of especially high relevance in forested landscapes (Haddad et al. 2015). Across Central Europe, forest and woodland management has changed strongly over the past 200 years (Kirby and Watkins 2015). With the arrival of coal, oil and gas, the demand for firewood both for industrial and domestic use has been declining since 1800 (McGrath et al. 2015). In the same period, forest use intensity declined: Coppice forests with short rotation cycles were converted into high forest at a large scale (Kirby and Watkins 2015; Kamp 2022), wood pastures were abandoned and afforested, and livestock grazing disappeared from forests in most regions (Plieninger et al. 2015). During the past 50 years, “close-to-nature” silviculture meant an abandonment of clear-cutting practices, additionally resulting in more continuous forest cover over longer periods (Palmero-Iniesta et al. 2021).
The decrease in biomass harvest from forests over the past 200 years (Schelhaas et al. 2003; McGrath et al. 2015) has had important, yet only partly quantified consequences for biodiversity. Historical, open coppice and grazed forests promoted the occurrence of many warm-adapted species that prefer light conditions (Vacik et al. 2009; Weiss et al. 2021). With an increase in biomass, tree-cover and tree-height, and an associated cooler and darker forest microclimate, many of these species have been declining dramatically over the past century (Hodgson et al. 2009; Swanson et al. 2011; Hilmers et al. 2018). Recently declines of birds (Gregory et al. 2019; Burns et al. 2021; Kamp et al. 2021) and insects (e.g. Seibold et al. 2019) in European forests over the past decades are at least partly attributable to the abovementioned changes in forest management (e.g. Thorn et al. 2015; Roth et al. 2021; Laussmann et al. 2021; Habel et al. 2022). However, changes in forest management have rarely been discussed as drivers of long-term insect declines (e.g. Wagner 2020).
A forest management type that was especially affected by large area losses and associated fragmentation is coppice, both with and without (henceforth “simple coppice”) standards (Buckley 2020; Slach et al. 2021; Kamp 2022). Afforestation of coppice with fast-growing conifers or European beech, but also coppice abandonment, resulted in fast canopy closing with cooler and darker stand conditions. Along with habitat loss, fragmentation of coppice was widespread in the process of transformation to high forest (Deconchat and Balent 2001), as usually the remotest and least productive areas were transformed or abandoned first, and as ownership structures are often complex (Kamp 2022).
Despite these dramatic reductions in coppice area, little is known about biodiversity responses to this century-long coppice loss. Traditionally coppiced woodlands create a small-scale mosaic of different successional stages and therefore provide habitats for different species and their individual requirements (Hilmers et al. 2018). Compelling evidence for community changes and abundance declines due to coppice loss and abandonment is available for plants (Kopecký et al. 2013) and birds (especially long-distance migrants, Fuller and Rothery 2013). Therefore, coppice loss is considered a major driver of the decline of woodland butterflies in Europe (Warren and Key 1991; Bergman 2001; van Swaay et al. 2006). Remaining coppice is considered as a highly valuable habitat especially for butterflies and moths (Fartmann et al. 2013; Merckx et al. 2012; Roth et al. 2021) as well as saproxylic beetles (Weiss et al. 2021). Coppice restoration and targeted management can lead to biodiversity gains, e.g. in spiders (Vymazalová et al. 2021), moths and butterflies (Hodgson et al. 2009; Dolek et al. 2018a; Merckx et al. 2012; Roth et al. 2021).
Much less is known about the effects of fragmentation, i.e. increasing isolation of remaining, used coupes on biodiversity, and on the relative contributions of habitat quality on occurrence and abundance of animals and plants in coppice. Interactions with important components of coppice systems, e.g. high Red Deer Cervus elaphus densities (Feber 2001; Spitzer et al. 2008; Ramirez et al. 2019), have also rarely been considered.
We used two butterfly species, Satyrium ilicis (Ilex Hairstreak) and Melitaea athalia (Heath Fritillary), that show clear preference for coppice forests in Central and Western Europe (Warren 1991; Hermann and Steiner 2000; Hodgson et al. 2009; Maes et al. 2014), as model species. We aimed to evaluate whether their populations are currently driven by patch size and fragmentation of remaining coppice patches (= coupes), or by habitat quality and successional stage, as other studies suggest (Warren 1987a, b; Brereton 2006; Ulrich and Caspari 2007; Bräu et al. 2013; Maes et al. 2014). Our main objective was to provide evidence for coppice managers and conservationists about the most important factors to consider when maintaining, managing and restoring coppice. We surveyed these species in what is perhaps the last intact, large-scale remaining simple coppice landscape in Central Europe (Kamp 2022).
We hypothesized that butterfly densities:
-
1.
increase with increasing patch (= coupe) size and increasing patch connectivity, because larger patches exhibit a higher resource availability, and isolation impairs colonization of empty patches,
-
2.
increase with warmer microclimate, because coppice insects are warm-adapted species,
-
3.
peak at species-specific successional stages, because these provide a maximum of resources for larval and adult stages,
-
4.
increase with an increasing habitat quality, i.e. an increasing cover of larval food and nectar plants,
-
5.
increase with increasing intensity of Red Deer browsing, that delays the succession of woody species and thus the development of a closed canopy.
Materials and methods
Study area
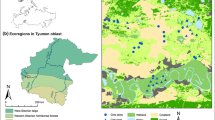
The study region is located in the federal states of Hesse and North Rhine-Westphalia (NRW) in Germany, centred on 50°47’ N, 8°14’ E and 50°58’ N, 8°07’ E. It forms the largest remaining areas of simple coppice (coppice-without-standards) in Germany and is one of the largest in Central Europe. The total size of the study area is 14,000 ha (situated between 300 and 600 m a.s.l.), with about 5000 ha active coppice (Kamp 2022). In Hesse, coupes are larger and rotation cycles of recurring cutting are shorter than in North Rhine-Westphalia (Kamp 2022). The parcels are mainly located on slopes and the coppices grow on relatively nutrient-poor acidic soils (Podzols and Cambisols) (Stegger and Vinnemann 2013). Alongside coppice, managed high beech forests and Norway spruce plantations are found in the study area. The climate is characterised by an annual precipitation of 750–950 mm and a mean annual temperature of 8.9 to 9.7 °C (Deutscher Wetterdienst (DWD) 2017).
Location of the study area in Germany with all remaining active coppice coupes (yellow), afforested coppice (dark green) and abandoned coppice (light green) (Kamp 2022). Investigated coupes are marked with different symbols
Coupes are sparsely vegetated in the first two years after cutting. A shrubby stage persists from the third to the ninth year that is dominated by resprouting birch (Betula pendula et pubescence), scotch broom (Sarothamnus scoparius) and heather (Calluna vulgaris). Approximately 15 years after cutting, the canopy closes to stands dominated by oak (Quercus petraea et robur) and birch, with bilberry (Vaccinium myrtillus) carpets in the dwarf-shrub layer (Suppl. Material Fig. S1). The study area is also characterised by intensive gamekeeping with very high densities of Red Deer (Cervus elaphus), Roe Deer (Capreolus capreolus) and Wild Boar (Sus scrofa), that find optimal habitat in coppice forests (Joys et al. 2004; Benes et al. 2006).
Butterfly surveys
Information on coupe size and the year of the last cut was taken from an area-wide database that was assembled from high-resolution remote sensing data (Kamp 2022). In the study area, coupes that were cut 1–9 years ago were considered as suitable habitat, since early and mid-successional woodland stages are primarily colonized by S. ilicis and M. athalia (Fartmann et al. 2013; Maes et al. 2014). In 2018, 86 coppice woodland patches were selected randomly in Hesse, stratified by size, isolation and stand age of the coupes. In the following year, we revisited 38 out of the 86 patches and added 44 new patches, 4 fresh cuts in Hesse (1 year after cutting) and 40 plots in NRW (1–9 years after cutting) (Fig. 1). The single coupes varied in size between 0.32 and 17.77 ha (mean ± SD: 5.10 ± 4.18) in Hesse and between 0.38 and 5.42 ha (mean ± SD: 1.73 ± 1.17) in NRW. At each coupe, a starting point was chosen randomly (with a buffer of 50 m to the edge to avoid strong edge effects), and a transect was walked set from this starting point dissecting the coupe in the direction of the slope. Due to varying coupe size, transects varied in length from 40 to 455 m (mean ± SD: 161.82 ± 94.21).
We surveyed all transects for adult individuals of S. ilicis and M. athalia five times per year between late May and mid-July in 2018 and 2019. To correct for varying factors that affect detectability, and to predict population densities without labour-intensive capture-recapture efforts, we used Distance Sampling rather than simple Pollard walks (Buckland et al. 2008; Isaac et al. 2011). Observations were assigned to five distance classes (0–2.5 m, 2.5–5 m, 5–10 m, 10–20 m, > 20 m) on both sides of the transect line. We identified S. ilicis and M. athalia with the aid of binoculars. Occasionally, hand-held nets were used to catch individuals and release them afterwards. Surveys took place between 9:00 a.m. and 7:00 p.m. (GTM + 2), at wind speeds below 4 Bft. and temperatures > 17 °C when the sky was clear, at least 20 °C at less than 40% cloud cover, and at least 25 °C when overcast.
Habitat quality
To assess habitat quality for S. ilicis and M. athalia we collected information on parameters known to influence their occurrence and abundance. In late June of each survey year, parameters were recorded at the beginning, midpoint and end of each transect, in a quadrat of 10 × 10 m. Endpoints were placed 10 m ahead of the end of the transect to avoid strong edge effects. For analysis, measurements were averaged over the three recording points. We measured cover and heights of herb, shrub and tree layer for each coupe. To assess the availability of preferred host plants for the larvae and nectar source for butterflies, we estimated the cover of Quercus spp. (both Quercus robur and Quercus petraea) in the area, which is the foodplant of S. ilicis, Melampyrum pratense and Digitalis purpurea, which are larval foodplants of M. athalia, and Rubus fruticosus agg. that is a key nectar resource for both species in the area. We estimated relative Red Deer browsing intensity by counting dung piles at a strip of 2 m width along the transects, and later calculated dung density per 100 m2 (Marques et al. 2001).
For each plot, elevation, slope and aspect were calculated from a digital elevation model (U.S./Japan ASTER Science Team 2011) in QGIS Version 3.14 and then used to calculate a heat load index following McCune (2007).
Habitat fragmentation and landscape matrix
To evaluate the fragmentation of coppice on a landscape scale the area of each sampled coupe and that of neighbouring coupes suitable for colonization (stand age 1–9 years) within a 2 km radius were determined. Together with the Euclidean distance from the edge of each coupe to all edges of neighbouring coupes, measured by the use of R package “rgeos” (Bivand et al. 2017), we calculated the habitat connectivity (I) of each sampled coupe (i) by the following formula:
with Aj being the size of the neighbouring coupe j and dij the Euclidian distance from the focal coupe i to the neighbouring coupe j (Steffan-Dewenter and Tscharntke 2000). We used Euclidian distance instead of functional distance to calculate connectivity because of the lack of species-specific information on resistance values for landscape elements (Poniatowski et al. 2016). A high value of I indicates high connectivity and low fragmentation. To characterise the surrounding landscape of each coupe, the percentage cover of high forest, transitional woodland/shrub and grassland/pastures within a 1000 m radius of the coupe was determined (Berg et al. 2011) based on Corine Land Cover 2018 (European Union, Copernicus Land Monitoring Service 2019).
Data analysis
All statistical analyses were performed in R version 4.1.3 (R Core Team 2022). We first modelled butterfly densities of adult individuals for each sampling site with Hierarchical Distance Sampling models (Royle et al. 2004) using the function gdistsamp of the package “unmarked” (Fiske et al. 2015). We used a half-normal detection probability function because we had comparatively few distance classes. We tested variables influencing the detectability of butterflies (e.g. weather, observer) in the detection part of the gdistsamp function. As models containing these were consistently not selected as best models, we decided not to include covariates in the detection part of the model. We also estimated abundance independently of covariates, i.e. with an intercept-only model. For subsequent analyses, we used the estimated density of that survey round that yielded the highest density (i.e. represented the phenological peak of the flight season).
Since hierarchical Distance Sampling models do not easily allow for an inclusion of random effects, but as this was necessary due to spatial dependencies in the data, we extracted transect-level predicted densities of both species and related them in a following step to explanatory variables. To predict density as a function of habitat parameters and isolation, we used Generalised Linear Mixed-effects Models (GLMM) fitted with functions of the “glmmTMB” package (Magnusson et al. 2017). The response variable, modelled population density per transect, is a continuous variable. We compared the performance and goodness-of-fit of models assuming different distributions (gaussian, gamma, tweedie) using the Aikaike information criterion (AICc) and a visual inspection of the residuals. Models using the tweedie distribution performed consistently better than those assuming other distributions, therefore we fitted the final models with this distribution. We incorporated study sites nested in communal district as a random effect in all models to account for district-level spatial dependencies among samples that are due to pronounced district-level variation in coppice management (Kamp 2022) and variation in topography and geography. Explanatory variables were standardised and also entered as squared variables to allow for hump-shaped relationships. To avoid multicollinearity, only one of a pair of correlated variables (Spearman´s rho >|0.6|) was allowed in the same model. We used the “DHARMa” package to evaluate the models and to test if model assumptions were met (Hartig 2020). To compare the models the function model.sel of the package “MuMln” was used (Bartoń 2022). Conditional and marginal R2 were calculated following Nakagawa and Schielzeth (2013). For predictions, the function ggpredict in the package “ggeffects” (Lüdecke et al. 2022) was applied. Marginal effects (i.e. prediction when keeping all other model variables at their mean) of explanatory variables were visualized. We used GLMMs without covariates and coupes nested in communal district as a random effect (with tweedie-distribution) to predict mean density during flight maximum and total population size per species across the investigated coupes. To avoid spurious results and data dredging, we specified a number of separate full models based on the hypotheses outlined in the introduction. We compared these models using AICC and Akaike weights, as they were all fitted on the same dataset.
A Principal Component Analysis (PCA) was used (function prcomp in package “stats”) to visualise the relationship between explanatory habitat variables.
Results
Occurrence, density and population size
In 2018, M. athalia was detected at 18 and S. ilicis at 52 coupes (age 1–9 years, N = 86 transects, Suppl. Material Fig. S2). M. athalia occurred during the first two sampling occasions and S. ilicis during the entire sampling period (Fig. 5). In 2019, M. athalia was detected at 25 coupes and S. ilicis at 35 coupes (N = 82 transects), flight periods were later than in 2018 (Table 1; Fig. 5, Suppl. Material Fig. S2). Estimated densities for adult individuals and total population sizes were higher for S. ilicis than for M. athalia (Table 1).
Environmental conditions across the surveyed coppice coupes
During the first two years after clear-cutting, the coupes were characterized by much open soil, dead wood and a low shrub and tree layer. In mid-successional stages (year 3–6) shrub layer cover peaked, but there was still a distinct grass and herb layer. In the late successional stages (year 7–9), tree cover increased, and the canopy started to close. Fewer shrubs were present (Fig. 2, Suppl. Material Fig. S3, Table S4).
Principal component analysis of habitat parameters. Light blue: early successional stages (1–2 years since last cut); yellow: intermediate stages (3–6 years since last cut); green: late successional stages (7–9 years since last cut). PC1 (Eigenvalue 3.36) and PC2 (Eigenvalue 2.96) together explained 33% of the variance in the data. Habitat parameters with a regression coefficient >|0.8| along the first two axes are depicted. Circles show coupes with M. athalia present, squares show coupes with S. ilicis present, rotated squares show coupes with both species and triangles show stands with neither species present. Red plus signs show estimated correlation values of each variable with PC1 and PC2 (see also Suppl. Material Table S5)
Habitat models
Neither patch (= coupe) connectivity nor landscape permeability were significant predictors of the density of M. athalia and S. ilicis (Table 2; Fig. 3). A higher density of S. ilicis, but not M. athalia was associated with a higher heat load (Fig. 4). Both species showed a humped-shaped relationship with time since last cut, for S. ilicis the model containing this variable receiving by far the highest support in comparison to all other models (Table 2; Fig. 5). S. ilicis increased in abundance with an increase in oak cover and showed highest predicted densities at mean shrub heights between 100 and 130 cm (Fig. 6). M. athalia densities were positively related to the cover of its larval food plant (Melampyrum pratense) (Table 2; Fig. 6). The intensity of Red Deer browsing had a significantly positive effect on S. ilicis (Table 2), but non on M. athalia (Fig. 7).
Model comparisons suggested that for S. ilicis, stand age (time since last cut, model S.i.3) explained most of the variation in the data given a certain model complexity based on AICc values (Table 2). For M. athalia, in all three models, the stand age model (model M.a.3), the microclimate model (model M.a.2) and the larval food plant model (model M.a.4) had similar fit based on AICc values (ΔAICc ≤ 2, Table 2).
Predicted densities (marginal means ± 95% confidence intervals) of the two butterfly species (a) S. ilicis and (b) M. athalia as a function of coupe connectivity, from models S.i.1 and M.a.1 in Table 2 (effect not significant). All other covariates of the model were kept constant at their mean
Modelled densities (marginal means ± 95% confidence interval) of the two butterfly species (a) S. ilicis and (b) M. athalia predicted as a function of the plot-level heat load index, from models S.i.2 and M.a.2 in Table 2 (only significant for M. athalia). All other covariates of the model were kept constant at their mean
Variation in predicted densities (marginal means ± SE) as a function of stand age (years since last cut) across all sampled coupes, separately per year and study region, for S. ilicis (a, b, c) and M. athalia (d, e, f). Phenological variation over the annual sampling periods is also illustrated, with dark blue corresponding to the first survey round, light blue to the second, orange to the third, red to the fourth and brown to the fifth round. All other covariates of the model were kept constant at their mean
Predicted densities (marginal means ± 95% confidence interval) of S. ilicis (a, b) and M. athalia (c, d) predicted as a function of the plot-level larval foodplant cover (%), from models S.i.4 and M.a.4 in Table 2. All other covariates of the model were kept constant at their mean
Predicted densities (marginal means ± 95% confidence interval) of S. ilicis (a) and M. athalia (b) as a function of Red Deer browsing intensity, from models S.i.5 and M.a.5 in Table 2. All other covariates of the model were kept constant at their mean
Discussion
We showed that one of the last intact simple coppice landscapes of Central Europe provides high-quality habitat for M. athalia and S. ilicis and hosts large populations. The total population size of S. ilicis during the flight maximum across the survey coupes was predicted to be 7503 individuals in 2018 and 3486 individuals in 2019. For M. athalia, the total number of individuals predicted was 1805 in 2018 and 2235 in 2019 across the surveyed coupes. Occurrence and numbers of individuals were strongly determined by habitat quality and changes therein along a successional gradient, represented by the stand age, i.e. time since last cut. Population density of M. athalia imagines increased with increasing cover of the primary larval host plant Melampyrum pratense but was negatively affected by a high heat load index. S. ilicis population density was positively associated with the intensity of Red Deer browsing, as well as increasing cover and a specific height of oak trees resprouting from stools as host plants for the larvae. Some of the phenological differences between 2018 and 2019 may be due to the fact that the spring of 2018 was drier and warmer than 2019. Both species showed an earlier flight period and higher densities in 2018 (Hesse), indicating the sensitivity of butterfly populations to individual weather events and climatic variations (Badik et al. 2015).
Coupe connectivity showed no measurable effect on modelled densities at flight maximum for either species. Because the availability of suitable habitat in the landscape is still relatively high across our study area, the importance of patch isolation and connectivity might be of secondary importance to the species here (Krauss et al. 2003; Bergman et al. 2004; Krämer et al. 2012; Merckx et al. 2019). The higher importance of habitat quality over isolation and patch size was also emphasized in other studies (Fleishman et al. 2002; van Halder et al. 2015; Poniatowski et al. 2018) and shown for other butterfly species (Thomas et al. 2011; Krämer et al. 2012; Fartmann et al. 2013).
Climate and weather have a substantial impact on all development stages of butterflies (Radchuk et al. 2013); sometimes even single weather events can affect populations heavily (Lynch et al. 2014; Badik et al. 2015). Consequently, the microclimatic conditions are an important factor influencing habitat quality, which is crucial for the continued survival of metapopulations. Other studies have suggested that S. ilicis prefers warm microclimate conditions (Maes et al. 2014). In our study, such a relationship was not detectable. M. athalia showed lower densities in areas of a high heat load index. This indicates that the species can make better use of steeper and north-exposed, and therefore cooler slopes. The decreasing density with higher heat stress can be interpreted as an indication of not coping optimally with hot environments, for example early successional stages, and might make the species vulnerable to climate change. About five to six years after the last cut, total cover and denser vegetation create a cooler microclimate, as also Fartmann et al. (2013) found in coppice with standards in French Alsace. In contrast, in south eastern England, where the climate is oceanic, populations of M. athalia in coppice forests showed a clear preference for sunny and warm sites (Warren 1987a).
The abundance of both species peaked at a stand age (time since last cut) of ca. four to six years, illustrating the high importance of open coppice during early-succession. Stand age reflects a certain habitat quality, as both vegetation composition, its height and density, and microclimate change with progressing succession. M. athalia reached highest densities somewhat later than S. ilicis, as also shown in other studies (Treiber 2003). This successional stage is characterised by a rich supply of young oak and birch shrubs, which, however, do not yet dominate and allow for a rich herb layer, where some shaded patches already exist. In later stages of succession after about eight years, habitat quality decreases (Warren and Key 1991; Strätling 2010; Blixt et al. 2015). Other studies also point to peaking butterfly and moth abundances in successional coppice habitats between two and seven years after clear-cut/disturbance (Warren 1987b; Merckx et al. 2012; Fartmann et al. 2013; Dolek et al. 2018b), suggesting that our results might be generalizable for coppice-dependent butterfly species. Habitat characteristics of stands of the same age, in addition to influences from herbivory (Benes et al. 2006), can vary due to geographic location and therefore show different levels of succession. In comparison to our findings, coppice systems in the UK investigated by Warren (1987b) showed a closed canopy after five to six years and highest abundances of M. athalia in stands of age two to four years. Our study site in Germany showed a closing canopy after ten to twelve years, indicating slower succession processes. As a consequence, highest predicted abundances were found in comparatively old stands. The peaking of S. ilicis abundances in early successional stages reflects its clear preference for low-growing open vegetation (Treiber 2003; Strätling 2010; Maes et al. 2014). The traditional, regular management of coppice woodlands in the studied low mountain range of Central Germany is of central importance for M. athalia and S. ilicis. The annual cutting of areas of varying size creates a heterogeneous mosaic of different succession stages. A sufficient supply of suitable areas nearby is essential for the survival of many light forest/woodland butterflies (Warren and Thomas 1992; Brereton 2006; Twardella and Fasel 2007; Hodgson et al. 2009; Merckx and Berwaerts 2010).
Especially species with monophagous larval stages are strongly bound to their host plants (Krauss et al. 2005; Krämer et al. 2012; van Halder et al. 2015). In general, the occurrence and abundance of many species is particularly influenced by the requirements of the preimaginal stages, as they are little mobile and are mostly relatively long-lived in comparison to the adult butterfly (Fartmann and Hermann 2006; Merckx and Berwaerts 2010; Thomas et al. 2011). For M. athalia, a high availability of the larval food plant allowed high densities of the imagines in our study. The availability of Digitalis purpurea, as a food plant for the later larval stages, was not a significant predictor of adult population density. Despite the low average cover of M. pratense, considerably higher M. athalia densities were predicted even at comparatively little increase of cover. M. pratense thus acts as a strong limiting factor in our populations, as also shown by Warren et al. (1984). Oak cover was not among the most important parameters in explaining the density of S. ilicis. This may be due to the generally high stand densities of oaks in the study area and the huge number of resprouting oak stools (likely hundreds of thousands). The higher densities of S. ilicis at shrub heights between 100 and 130 cm point towards the importance of a certain successional stage for the species, as females of the species prefer low, shrubby oaks for oviposition (Ulrich and Caspari 2007).
Herbivory by large mammals can affect the habitat quality for insects by retarding vegetation succession. Game browsing occurs primarily in palatable early and light successional stages (Treiber 2003; Joys et al. 2004; Hédl et al. 2010). Therefore, the influence of browsing animals after coppice abandonment may be crucial for the maintenance of light forest structures (Benes et al. 2006). In the coppice of our study area, regular Red Deer browsing keeps the oak trees in a bushy stage, and therefore in a suitable condition as host plants for S. ilicis larvae (Köstler 2005). The oviposition occurs preferably on young and small oak trees, often less than 50 cm in height (Koschuh and Fauster 2005; Maes et al. 2014). However, conditions may change when intense browsing decreases the amount of fresh leaves and buds, which are the food source for caterpillars (Koschuh and Fauster 2005; Maes et al. 2014). Ulrich and Caspari (2007) observed that heavily browsed gnarled central shoots were even preferentially approached by S. ilicis for oviposition. This is in line with our results of increasing population density of S. ilicis with increasing Red Deer density. The present study confirms earlier studies concluding that S. ilicis benefits from a high density of browsers that maintain a certain habitat quality (Schiess-Bühler 2004; Hermann 2007; Ulrich and Caspari 2007; FVA-BW 2022).
For M. athalia, on the other hand, there was no detectable influence of browsing intensity on population density of the imagines. High Red Deer browsing pressure results in grass-rich stands (Gill and Beardall 2001; Kirby 2001; Gill and Fuller 2007) at the cost of dwarf shrubs such as Calluna vulgaris and bilberry (Vaccinium myrtillus). Bilberry carpets often host larger populations of M. athalia’s larval food plant, Melampyrum pratense that is hemiparasite on bilberry. M. pratense is not directly grazed but sensitive to trampling (Klotz and Kühn 2002). Intensive Red Deer foraging can also lead to damage of nectar plants and therefore can have a negative impact on butterflies (Feber 2001, Boulanger et al. 2018).
Conclusions
As characteristic species of light, open forest M. athalia and S. ilicis, reflect the high conservation value of young forest succession stages that also provide habitat for many other insect species (Freese et al. 2006; Merckx et al. 2012; Fartmann et al. 2013). Due to the decline of light-demanding insects in forests, the preservation of open forest systems with a mosaic of successional stages, such as coppice, is of particular importance (Freese et al. 2006; van Swaay et al. 2006; Benes et al. 2006; Vacik et al. 2009). Coppice can also serve as a biodiversity-enhancing buffer zone between high forest and open land (Merckx et al. 2012). Because of the preference for early successional stages in both species, a transformation of the studied coppice systems to spruce plantations or high beech forest would likely lead to a disappearance of both species in the area.
Conservation managers should aim to maintain coppice systems with large number of moderately isolated, large coupes of several hectares in size as in our study area, because the effects of fragmentation seem to be less important in such “intact” coppice systems. Habitat quality should be maintained through short rotation cycles that always provide a supply of well-connected, four- to six-year-old, large, continuous coupes, benefitting both species. For S. ilicis, a certain level of Red Deer browsing is beneficial. For M. athalia, very high Red Deer densities should be avoided. Climate change may reduce the extent of habitat that meets the niche requirements of M. athalia, as this species appeared to be a more cold-adapted in our study, suggesting that maintaining open forests with a high microclimatic habitat diversity in terms of slope and exposition is even more important.
References
Badik KJ, Shapiro AM, Bonilla MM et al (2015) Beyond annual and seasonal averages: using temporal patterns of precipitation to predict butterfly richness across an elevational gradient. Ecol Entomol 40:585–595. https://doi.org/10.1111/een.12228
Bartoń K (2022) MuMIn: Multi-Model Inference. Version 1.46.0URL https://CRAN.R-project.org/package=MuMIn
Benes J, Cizek O, Dovala J, Konvicka M (2006) Intensive game keeping, coppicing and butterflies: the story of Milovicky Wood, Czech Republic. For Ecol Manag 237:353–365. https://doi.org/10.1016/j.foreco.2006.09.058
Berg Ã, Ahrné K, Öckinger E et al (2011) Butterfly distribution and abundance is affected by variation in the swedish forest-farmland landscape. Biol Conserv 144:2819–2831. https://doi.org/10.1016/j.biocon.2011.07.035
Bergman K-O (2001) Population dynamics and the importance of habitat management for conservation of the butterfly Lopinga achine. J Appl Ecol 38:1303–1313. https://doi.org/10.1046/j.0021-8901.2001.00672.x
Bergman K-O, Askling J, Ekberg O et al (2004) Landscape effects on butterfly assemblages in an agricultural region. Ecography 27:619–628. https://doi.org/10.1111/j.0906-7590.2004.03906.x
Bivand R, Rundel C, Pebesma E et al (2017) Package ‘rgeos’
Blixt T, Bergman K-O, Milberg P et al (2015) Clear-cuts in production forests: from matrix to neo-habitat for butterflies. Acta Oecol 69:71–77. https://doi.org/10.1016/j.actao.2015.09.006
Boulanger V, Dupouey J, Archaux F, et al (2018) Ungulates increase forest plant species richness to the benefit of non‐forest specialists. Glob Chang Biol 24:485–495. https://doi.org/10.1111/gcb.13899
Bräu M, Bolz R, Kolbeck H et al (2013) Tagfalter in Bayern. Verlag Eugen Ulmer, Stuttgart
Brereton T (2006) Monitoring the Heath Fritillary Mellicta athalia in Thornden and West Blean Woods. Monitoring Nature Conservation in Cultural Habitats. Springer, Dordrecht, pp 271–284
Buckland ST, Marsden SJ, Green RE (2008) Estimating bird abundance: making methods work. Bird Conserv Int 18:91–108. https://doi.org/10.1017/S0959270908000294
Buckley P (2020) Coppice restoration and conservation: a european perspective. J For Res 25:125–133. https://doi.org/10.1080/13416979.2020.1763554
Burns F, Eaton MA, Burfield IJ et al (2021) Abundance decline in the avifauna of the European Union reveals cross-continental similarities in biodiversity change. Ecol Evol 11:16647–16660. https://doi.org/10.1002/ece3.8282
R Core Team (2022) R: a language and environment for statistical computing
Deconchat M, Balent G (2001) Vegetation and bird community dynamics in fragmented coppice forests. For Int J For Res 74:105–118. https://doi.org/10.1093/forestry/74.2.105
Deutscher Wetterdienst (DWD) (2017) Temperatur: vieljährige Mittelwerte 1981–2010 und Niederschlag: vieljährige Mittelwerte 1981–2010. In: Vieljährige Mittelwerte 1981–2010. https://www.dwd.de/DE/leistungen/klimadatendeutschland/mittelwerte/temp_8110_fest_html.html?view=nasPublication. Accessed 21 Feb 2019
Dolek M, Kőrösi Á, Freese-Hager A (2018a) Successful maintenance of Lepidoptera by government-funded management of coppiced forests. J Nat Conserv 43:75–84. https://doi.org/10.1016/j.jnc.2018.02.001
Dolek M, Kőrösi Á, Freese-Hager A (2018b) Successful maintenance of Lepidoptera by government-funded management of coppiced forests. J Nat Conserv 43:75–84. https://doi.org/10.1016/j.jnc.2018.02.001
European Union, Copernicus Land Monitoring Service (2019) Corine Land Cover Dataset 2018
Fahrig L (2003) Effects of Habitat Fragmentation on Biodiversity. Annu Rev Ecol Evol Syst 34:487–515. https://doi.org/10.1146/annurev.ecolsys.34.011802.132419
Fahrig L (2017) Ecological responses to Habitat Fragmentation per Se. Annu Rev Ecol Evol Syst 48:1–23. https://doi.org/10.1146/annurev-ecolsys-110316-022612
Fahrig L, Arroyo-Rodríguez V, Bennett JR et al (2019) Is habitat fragmentation bad for biodiversity? Biol Conserv 230:179–186. https://doi.org/10.1016/j.biocon.2018.12.026
Fartmann T, Hermann G (2006) Larvalökologie von Tagfaltern und Widderchen in mitteleuropa–von den Anfängen bis heute. Abh Westf Mus Naturk 68:11–57
Fartmann T, Müller C, Poniatowski D (2013) Effects of coppicing on butterfly communities of woodlands. Biol Conserv 159:396–404. https://doi.org/10.1016/j.biocon.2012.11.024
Feber RE (2001) The impacts of deer on woodland butterflies: the good, the bad and the complex. Forestry 74:271–276. https://doi.org/10.1093/forestry/74.3.271
Fischer J, Lindenmayer DB (2007) Landscape modification and habitat fragmentation: a synthesis. Glob Ecol Biogeogr 16:265–280. https://doi.org/10.1111/j.1466-8238.2007.00287.x
Fiske I, Chandler R, Miller D et al (2015) Package ‘unmarked’. R Proj Stat Comput
Fleishman E, Ray C, Sjögren-Gulve P et al (2002) Assessing the roles of patch quality, area, and isolation in predicting metapopulation dynamics. Conserv Biol 16:706–716. https://doi.org/10.1046/j.1523-1739.2002.00539.x
Fletcher RJ, Didham RK, Banks-Leite C et al (2018) Is habitat fragmentation good for biodiversity? Biol Conserv 226:9–15. https://doi.org/10.1016/j.biocon.2018.07.022
Freese A, Benes J, Bolz R et al (2006) Habitat use of the endangered butterfly Euphydryas maturna and forestry in Central Europe. Anim Conserv 9:388–397. https://doi.org/10.1111/j.1469-1795.2006.00045.x
Fuller RJ, Rothery P (2013) Temporal consistency in fine-scale habitat relationships of woodland birds during a period of habitat deterioration. For Ecol Manag 289:164–174. https://doi.org/10.1016/j.foreco.2012.09.035
FVA-BW (2022) Brauner Eichen-Zipfelfalter - Waldnaturschutz-Informationssystem Baden-Württemberg. https://wnsinfo.fva-bw.de/arten/brauner-eichen-zipfelfalter. Accessed 11 Jul 2022
Gill RMA, Beardall V (2001) The impact of deer on woodlands: the effects of browsing and seed dispersal on vegetation structure and composition. For Int J For Res 74:209–218. https://doi.org/10.1093/forestry/74.3.209
Gill RM, Fuller RJ (2007) The effects of deer browsing on woodland structure and songbirds in lowland Britain. Ibis 149:119–127. https://doi.org/10.1111/j.1474-919X.2007.00731.x
Gregory R, Skorpilova J, Voříšek P, Butler S (2019) An analysis of trends, uncertainty and species selection shows contrasting trends of widespread forest and farmland birds in Europe. Ecol Indic 103:676–687. https://doi.org/10.1016/j.ecolind.2019.04.064
Habel JC, Schmitt T, Gros P, Ulrich W (2022) Breakpoints in butterfly decline in Central Europe over the last century. Sci Total Environ 851:158315. https://doi.org/10.1016/j.scitotenv.2022.158315
Haddad NM, Brudvig LA, Clobert J et al (2015) Habitat fragmentation and its lasting impact on Earth’s ecosystems. Sci Adv 1:e1500052. https://doi.org/10.1126/sciadv.1500052
Hartig F (2020) DHARMa: residual diagnostics for hierarchical (multi-level/mixed) regression models
Hédl R, Kopecký M, Komárek J (2010) Half a century of succession in a temperate oakwood: from species-rich community to mesic forest. Divers Distrib 16:267–276. https://doi.org/10.1111/j.1472-4642.2010.00637.x
Hermann G (2007) Tagfalter suchen im Winter-Searching for butterflies in Winter. Books on Demand GmbH, Norderstedt
Hermann G, Steiner R (2000) Satyrium ilicis in Baden-Württemberg: an example of an endangered light-demanding forest species. Naturschutz und Landschaftsplanung 32:271–277
Hilmers T, Friess N, Bässler C et al (2018) Biodiversity along temperate forest succession. J Appl Ecol 55:2756–2766. https://doi.org/10.1111/1365-2664.13238
Hodgson JA, Moilanen A, Bourn NAD et al (2009) Managing successional species: modelling the dependence of heath fritillary populations on the spatial distribution of woodland management. Biol Conserv 142:2743–2751. https://doi.org/10.1016/j.biocon.2009.07.005
Isaac NJ, Cruickshanks KL, Weddle AM et al (2011) Distance sampling and the challenge of monitoring butterfly populations. Methods Ecol Evol 2:585–594. https://doi.org/10.1111/j.2041-210X.2011.00109.x
Jaureguiberry P, Titeux N, Wiemers M et al (2022) The direct drivers of recent global anthropogenic biodiversity loss. Sci Adv 8:eabm0082. https://doi.org/10.1126/sciadv.abm9982
Joys AC, Fuller RJ, Dolman PM (2004) Influences of deer browsing, coppice history, and standard trees on the growth and development of vegetation structure in coppiced woods in lowland England. For Ecol Manag 202:23–37. https://doi.org/10.1016/j.foreco.2004.06.035
Kamp J (2022) Coppice loss and persistence in Germany. Trees For People 8:100227. https://doi.org/10.1016/j.tfp.2022.100227
Kamp J, Frank C, Trautmann S et al (2021) Population trends of common breeding birds in Germany 1990–2018. J Ornithol 162:1–15. https://doi.org/10.1007/s10336-020-01830-4
Kirby KJ (2001) The impact of deer on the ground flora of british broadleaved woodland. Forestry 74:219–229. https://doi.org/10.1093/forestry/74.3.219
Kirby K, Watkins C (2015) Europe’s changing woods and forests: from wildwood to managed landscapes. CABI Publishing, Oxfordshire, Boston
Klotz S, Kühn I (2002) Biolflor: Eine Datenbank zu biologisch-ökologischen Merkmalen zur Flora von Deutschland. In: Schriftenreihe Für Veg. Bonn. https://www.ufz.de/biolflor/taxonomie/taxonomie.jsp?ID_Taxonomie=1960. Accessed 16 Apr 2019
Kopecký M, Hédl R, Szabó P (2013) Non-random extinctions dominate plant community changes in abandoned coppices. J Appl Ecol 50:79–87. https://doi.org/10.1111/1365-2664.12010
Koschuh A, Fauster R (2005) Brauner Eichen-Zipfelfalter Satyrium ilicis (Esper, 1789)(Lepidoptera: Lycaenidae) in der Steiermark (Österreich). Beitr Zur Entomofaunist 6:65–86
Köstler W (2005) Das Eiablage-Verhalten des Eichenzipfelfalters Satyrium ilicis (ESPER, 1779) nördlich der Alpen. galathea 21:47–54
Krämer B, Poniatowski D, Fartmann T (2012) Effects of landscape and habitat quality on butterfly communities in pre-alpine calcareous grasslands. Biol Conserv 152:253–261. https://doi.org/10.1016/j.biocon.2012.03.038
Krauss J, Steffan-Dewenter I, Tscharntke T (2003) How does landscape context contribute to effects of habitat fragmentation on diversity and population density of butterflies? J Biogeogr 30:889–900. https://doi.org/10.1046/j.1365-2699.2003.00878.x
Krauss J, Steffan-Dewenter I, Müller CB, Tscharntke T (2005) Relative importance of resource quantity, isolation and habitat quality for landscape distribution of a monophagous butterfly. Ecography 28:465–474. https://doi.org/10.1111/j.0906-7590.2005.04201.x
Laussmann T, Dahl A, Radtke A (2021) Lost and found: 160 years of Lepidoptera observations in Wuppertal (Germany). J Insect Conserv 25:273–285. https://doi.org/10.1007/s10841-021-00296-w
Lüdecke D, Aust F, Crawley S, Ben-Shachar M (2022) Package ‘ggeffects’ R Package Version 1.1.4
Lynch HJ, Rhainds M, Calabrese JM et al (2014) How climate extremes—not means—define a species’ geographic range boundary via a demographic tipping point. Ecol Monogr 84:131–149. https://doi.org/10.1890/12-2235.1
Maes D, Jacobs I, Segers N et al (2014) A resource-based conservation approach for an endangered ecotone species: the Ilex Hairstreak (Satyrium ilicis) in Flanders (north Belgium). J Insect Conserv 18:939–950. https://doi.org/10.1007/s10841-014-9702-0
Magnusson A, Skaug H, Nielsen A et al (2017) Package ‘glmmtmb’ R Package Version 0.2. 0
Marques FF, Buckland ST, Goffin D et al (2001) Estimating deer abundance from line transect surveys of dung: sika deer in southern Scotland. J Appl Ecol 38:349–363
McCune B (2007) Improved estimates of incident radiation and heat load using non-parametric regression against topographic variables. J Veg Sci 18:751–754. https://doi.org/10.1111/j.1654-1103.2007.tb02590.x
McGrath MJ, Luyssaert S, Meyfroidt P et al (2015) Reconstructing european forest management from 1600 to 2010. Biogeosciences 12:4291–4316. https://doi.org/10.5194/bg-12-4291-2015
Merckx T, Berwaerts K (2010) What type of hedgerows do Brown hairstreak (Thecla betulae L.) butterflies prefer? Implications for european agricultural landscape conservation. Insect Conserv Divers 3:194–204. https://doi.org/10.1111/j.1752-4598.2010.00088.x
Merckx T, Feber RE, Hoare DJ et al (2012) Conserving threatened Lepidoptera: towards an effective woodland management policy in landscapes under intense human land-use. Biol Conserv 149:32–39. https://doi.org/10.1016/j.biocon.2012.02.005
Merckx T, Dantas de Miranda M, Pereira HM (2019) Habitat amount, not patch size and isolation, drives species richness of macro-moth communities in countryside landscapes. J Biogeogr 46:956–967. https://doi.org/10.1111/jbi.13544
Nakagawa S, Schielzeth H (2013) A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol Evol 4:133–142. https://doi.org/10.1111/j.2041-210x.2012.00261.x
Palmero-Iniesta M, Pino J, Pesquer L, Espelta JM (2021) Recent forest area increase in Europe: expanding and regenerating forests differ in their regional patterns, drivers and productivity trends. Eur J For Res 140:793–805. https://doi.org/10.1007/s10342-021-01366-z
Plieninger T, Hartel T, Martín-López B et al (2015) Wood-pastures of Europe: Geographic coverage, social–ecological values, conservation management, and policy implications. Biol Conserv 190:70–79. https://doi.org/10.1016/j.biocon.2015.05.014
Poniatowski D, Löffler F, Stuhldreher G et al (2016) Functional connectivity as an indicator for patch occupancy in grassland specialists. Ecol Indic 67:735–742. https://doi.org/10.1016/j.ecolind.2016.03.047
Poniatowski D, Stuhldreher G, Löffler F, Fartmann T (2018) Patch occupancy of grassland specialists: Habitat quality matters more than habitat connectivity. Biol Conserv 225:237–244. https://doi.org/10.1016/j.biocon.2018.07.018
Radchuk V, Turlure C, Schtickzelle N (2013) Each life stage matters: the importance of assessing the response to climate change over the complete life cycle in butterflies. J Anim Ecol 82:275–285. https://doi.org/10.1111/j.1365-2656.2012.02029.x
Ramirez JI, Jansen PA, den Ouden J et al (2019) Long-term effects of wild ungulates on the structure, composition and succession of temperate forests. For Ecol Manag 432:478–488. https://doi.org/10.1016/j.foreco.2018.09.049
Roth N, Hacker HH, Heidrich L et al (2021) Host specificity and species colouration mediate the regional decline of nocturnal moths in central european forests. Ecography 44:941–952. https://doi.org/10.1111/ecog.05522
Royle JA, Dawson DK, Bates S (2004) Modeling abundance effects in distance sampling. Ecology 85:1591–1597. https://doi.org/10.1890/03-3127
Schelhaas M-J, Nabuurs G-J, Schuck A (2003) Natural disturbances in the european forests in the 19th and 20th centuries. Glob Change Biol 9:1620–1633. https://doi.org/10.1046/j.1365-2486.2003.00684.x
Schiess-Bühler H (2004) Aktionsplan Brauner Eichenzipfelfalter (Satyrium ilicis). Artenschutzmassnahmen für gefährdete Tierarten im Kanton Zürich 3–16
Seibold S, Gossner MM, Simons NK et al (2019) Arthropod decline in grasslands and forests is associated with landscape-level drivers. Nature 574:671–674. https://doi.org/10.1038/s41586-019-1684-3
Silva DJ, Palmeirim AF, Santos-Filho M et al (2022) Habitat Quality, not Patch size, modulates lizard responses to Habitat loss and fragmentation in the Southwestern Amazon. J Herpetol 56:75–83. https://doi.org/10.1670/20-145
Slach T, Volarik D, Madera P (2021) Dwindling coppice woods in Central Europe-Disappearing natural and cultural heritage. For Ecol Manag 501:119687. https://doi.org/10.1016/j.foreco.2021.119687
Spitzer L, Konvicka M, Benes J et al (2008) Does closure of traditionally managed open woodlands threaten epigeic invertebrates? Effects of coppicing and high deer densities. Biol Conserv 141:827–837. https://doi.org/10.1016/j.biocon.2008.01.005
Steffan-Dewenter I, Tscharntke T (2000) Butterfly community structure in fragmented habitats. Ecol Lett 3:449–456. https://doi.org/10.1111/j.1461-0248.2000.00175.x
Stegger U, Vinnemann C (2013) Bodenübersichtskarte der Bundesrepublik Deutschland 1: 1000000 (BÜK 1000)
Strätling R (2010) Bestandserfassung von Satyrium ilicis (ESPER, 1779), Brauner Eichen-Zipfelfalter (Lepidoptera: Lycaenidae), im deutschen Teil des Warndts (Saarland) durch systematische Eisuche. DELATTINIA 35:435–454
Swanson ME, Franklin JF, Beschta RL et al (2011) The forgotten stage of forest succession: early-successional ecosystems on forest sites. Front Ecol Environ 9:117–125. https://doi.org/10.1890/090157
Thomas J, Simcox D, Hovestadt T (2011) Evidence based conservation of butterflies. J Insect Conserv 15:241–258. https://doi.org/10.1007/s10841-010-9341-z
Thorn S, Hacker HH, Seibold S et al (2015) Guild-specific responses of forest Lepidoptera highlight conservation-oriented forest management - implications from conifer-dominated forests. For Ecol Manag 337:41–47. https://doi.org/10.1016/j.foreco.2014.10.031
Treiber VR (2003) Genutzte Mittelwälder - Zentren der Artenvielfalt für Tagfalter und Widderchen im Südelsass: Nutzungsdynamik und Sukzession als Grundlage für ökologische Kontinuität. Naturschutz Landschaftsplanung 35(1):50–63
Twardella R, Fasel P (2007) Die großschmetterlinge (lepidoptera) im “historischen hauberg fellinghausen.” In: Landesamt für Natur, Umwelt und Verbraucherschutz Nordrhein-Westfalen (ed) Niederwälder in nordrhein-westfalen: beiträge zur Ökologie, geschichte und erhaltung. Galunder, Recklinghausen, pp 203–219
U.S./Japan ASTER Science Team (2011) ASTGTM: ASTER Global Digital Elevation Model V002. NASA EOSDIS land process DAAC USGS Earth Resour Obs Sci EROS Cent. https://doi.org/10.5067/ASTER/ASTGTM.002
Ulrich R, Caspari S (2007) Die Lichtwaldfalter im Saarland: erstes Modellprojekt im Warndt. Abh Delattinia 33:23–68
Vacik H, Zlatanov T, Trajkov P et al (2009) Role of coppice forests in maintaining forest biodiversity. Silva Balc 10:35–45
van Halder I, Barnagaud J-Y, Jactel H, Barbaro L (2015) Woodland habitat quality prevails over fragmentation for shaping butterfly diversity in deciduous forest remnants. For Ecol Manag 357:171–180. https://doi.org/10.1016/j.foreco.2015.08.025
van Swaay C, Warren M, Loïs G (2006) Biotope use and trends of european butterflies. J Insect Conserv 10:189–209. https://doi.org/10.1007/s10841-006-6293-4
Vymazalová P, Košulič O, Hamřík T et al (2021) Positive impact of traditional coppicing restoration on biodiversity of ground-dwelling spiders in a protected lowland forest. For Ecol Manag 490:119084. https://doi.org/10.1016/j.foreco.2021.119084
Wagner DL (2020) Insect declines in the Anthropocene. Annu Rev Entomol 65:457–480. https://doi.org/10.1146/annurev-ento-011019-025151
Warren MS (1987a) The ecology and conservation of the Heath Fritillary Butterfly, Mellicta athalia: 1. Host selection and phenology. J Appl Ecol 24:467–482. https://doi.org/10.2307/2403887
Warren MS (1987b) The ecology and conservation of the Heath Fritillary Butterfly, Mellicta athalia: 2. Adult population strucure and mobility. J Appl Ecol 24:483–498. https://doi.org/10.2307/2403888
Warren MS (1991) The successful conservation of an endangered species, the heath fritillary butterfly Mellicta athalia, in Britain. Biol Conserv 55:37–56. https://doi.org/10.1016/0006-3207(91)90004-S
Warren MS, Key RS (1991) Woodlands: past, Present and potential for insects. The conservation of insects and their habitats. Academic Press, London, pp 155–211
Warren MS, Thomas CD, Thomas JA (1984) The status of the health fritillary butterfly Mellicta athalia Rott. In Britain. Biol Conserv 29:287–305. https://doi.org/10.1016/0006-3207(84)90001-6
Warren MS, Thomas JA (1992) Butterfly responses to coppicing. In: Buckley GP (ed) Ecology and management of coppice woodlands. Chapman & Hall, London, pp 249–270
Weiss M, Kozel P, Zapletal M et al (2021) The effect of coppicing on insect biodiversity. Small-scale mosaics of successional stages drive community turnover. For Ecol Manag 483:118774. https://doi.org/10.1016/j.foreco.2020.118774
Acknowledgements
We are very grateful to the heads of the coppice management collectives of all villages in the coppice woods of the Lahn-Dill district, Hesse for allowing access to their area and providing a large amount of information, especially Johannes Eckhardt, Roger Weitzel, Dietmar Orth and Klaus-Dieter Schmidt. Harro Schäfer and Rolf Twardella provided valuable insight into historical and current coppice management and Lepidoptera communities of the study area. We thank the Hessische Landesamt für Naturschutz, Umwelt und Geologie and Landesbetrieb (HLNUG, HessenForst) and the Landesamt für Natur, Umwelt und Verbraucherschutz North Rhine-Westphalia (LANUV) for granting permits. Many thanks to Claudia Frank and Josef Kallmayer who gave statistical advice.
Funding
Open Access funding enabled and organized by Projekt DEAL. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
A.Graser: Conceptualization, methodology, validation, formal analysis, investigation, data curation, writing – original draft, writing – review & editing, visualization. M. Kelling: Investigation, data curation, writing – review & editing. R. Pabst: Formal analysis, investigation, data curation, writing – review & editing, visualization. M. Schulz: Investigation, data curation, writing – review & editing. N. Hölzel: Resources, writing – review & editing, supervision. J. Kamp: Conceptualization, methodology, writing – original draft, writing – review & editing, supervision, project administration.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Graser, A., Kelling, M., Pabst, R. et al. Habitat quality, not patch isolation, drives distribution and abundance of two light-demanding butterflies in fragmented coppice landscapes. J Insect Conserv 27, 743–758 (2023). https://doi.org/10.1007/s10841-023-00494-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-023-00494-8