Abstract
Diversity inventories are critical to creating accurate species range maps and estimating population sizes, which in turn lead to better informed landscape and wildlife management decisions. Metabarcoding has facilitated large-scale environmental diversity surveys. However, the use of a metabarcoding approach with bird feces to survey arthropod diversity is still relatively undeveloped. The aim of this study was to see if and how a metabarcoding approach with bird feces could contribute to a saproxylic Coleoptera survey of traditional insect traps. We compared two methods of surveying saproxylic Coleoptera diversity (metabarcoding birds feces and deploying traditional traps) over two elevations in a mountain system. The two methods caught different species and different levels of functional guild richness. The metabarcoding method successfully recorded both distinct and overlapping portions of diversity from traditional collections, and the approach was also effective in signaling the presence of both rare species and nine country records. Our results show that metabarcoding Passerine bird feces can be successful when used alongside traditional collection methods to capture a broad diversity of saproxylic Coleoptera. This method, however, has quantitative and qualitative limitations, including the inability to produce species abundance data as well as the generation of false positives and negatives due to biases within the metabarcoding pipeline.
Implications for insect conservation
As many terrestrial ecosystems lose insect diversity, insect diversity surveys are essential to understand the scope of the loss. Despite metabarcoding approach shortcomings, the declining costs and shorter survey and processing time required for this approach compared to traditional survey methods indicate that it can be a valuable addition to the toolkit for saproxylic Coleoptera diversity surveys.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Insect species make up 66% of all animal species found on earth (Zhang et al. 2011) and are conservatively estimated to provide more than 57 billion USD in economic value to the United States alone as both crop pollinators and vital building blocks for ecological functioning (Losey and Vaughan 2006). Recent studies have shown an alarming decline in terrestrial insect populations in the past half century (Dirzo et al. 2014; Sánchez-Bayo and Wyckhuys 2019; Møller 2020). For instance, one often cited study found a 75% reduction in flying insects in natural areas in Germany over the last 27 years (Hallmann et al. 2017). The stressors driving this decline of terrestrial insect populations are multifaceted and anthropomorphically derived and include pollution, the rise of industrial farming, climate change, and deforestation (Wagner et al. 2021).
Saproxylic Coleoptera [beetles that live or depend on dead or dying wood in some part of their lifecycle (Speight 1989)] are not immune to this trend. In Europe, roughly 11% of native saproxylic Coleoptera are considered threatened and a further 13% are considered near threatened (Nieto and Alexander 2010). The group is hyper-diverse with approximately 350,000–400,000 species worldwide (Storka et al. 2015). It is also functionally diverse and can be divided into multiple functional guilds within different life history stages including predatory, mycophagous, myxomycophagous (slime mold feeders), xylophagous, detritivorous, and parasitoid (Gimmel and Ferro 2018). This width and breadth of the taxonomic and functional variability of saproxylic Coleoptera have shown to be integral to nutrient cycling and food webs (Stokland et al. 2012; Gimmel and Ferro 2018). Many types of saproxylic Coleoptera play vital roles in the decomposition of dead wood through the digestion of polysaccharides and lignin with endosymbiotic fungi and/or bacteria (Micó et al. 2011; Strid et al. 2014; Hardersen and Zapponi 2017). Some saproxylic Coleoptera are restricted to a single host species, occupying distinct ecological niches (Milberg et al. 2014). In addition to comprising essential ecosystem scaffolding, some families and species provoke economic damage, and climate change and increasingly even-aged stands are expected to compound this damage in the future (Pedlar et al. 2019; Sommerfeld et al. 2021).
Due to the ecological and economical importance of this group, surveys and inventories of saproxylic Coleoptera are employed throughout the world (see Saint-Germain et al. 2006; Karpiński et al. 2021) but the vast diversity of Coleoptera can hinder classification by morphological methods (Piper et al. 2019). Species level taxonomic identification expertise in many arthropod families often takes years to master (Macfadyen et al. 2019). The larval morphology of many species are completely unknown (Kamiński et al. 2019; Staniec et al. 2014). Furthermore, identifying fully cryptic species, or species that “morphology fails to delimit” (Liu et al. 2020) is only possible using molecular technology (DeSalle et al. 2005). For example, in the last decade Brontispa longissima (Gestro, 1885) (Coleoptera: Chrysomelidae) and two species of wireworms (Coleoptera: Elateridae) were split into cryptic species complexes due to distinct differences in genomes and geographic distributions despite only subtle differences in morphologic distinctions (Takano et al. 2011; Andrews et al. 2020, respectively).
Surveys and inventories of saproxylic Coleoptera are most often carried out using traditional insect traps, such as malaise, flight intercept, and baited or attraction traps. These established sampling devises can suffer from shortcomings, including weather-related failures, human or animal disruption and/or sabotage, and difficulty of locating installed traps.
Therefore, to detect or monitor insects and other species of interest in a rapidly changing world, scientists are increasingly employing a metabarcoding approach to examine environmental diversity (Liu et al. 2020). Metabarcoding allows the simultaneous identification of taxa within bulk samples by the parallel sequencing of a portion of a gene (barcode) (Shokralla et al. 2012). Generally, DNA is extracted from a sample and a segment of a gene is amplified through PCR using a set of primers chosen with the organism or taxonomic group of interest in mind. The resulting amplicons are dual-tagged to facilitate re-assignment into the original samples and are pooled to form sequencing libraries. Finally, the amplicon sequence reads are assigned to taxonomic classifications using various bioinformatic methods (Piper et al. 2019; Liu et al. 2020). Over the last decade, this technology has advanced from a laboratory protocol (Yu et al. 2012) and enabled studies documenting arthropod diversity in bulk samples from a variety of habitats such as fresh water (Hajibabaei et al. 2019), caves (West et al. 2020), soil (Porter et al. 2019), and in bulk samples collected from traditional traps such as malaise traps (Hardulak et al. 2020).
Herein, we test a metabarcoding-based method to document saproxylic Coleoptera diversity in Passerine feces collected from birds captured and released from mist nets. Other studies have surveyed arthropod diversity in feces of birds caught in agricultural settings (Crisol-Martínez et al. 2016; Jedlicka et al. 2017), and in feces of nestlings (Rytkönen et al. 2019), and in feces in adults taken from nest boxes (Shutt et al. 2020, 2021). Ribeiro (2019) examined dietary niches of an African Passerine by both metabarcoding the birds’ feces and potential prey caught in pitfall traps.
Our aim was to ascertain whether metabarcoding bird feces could compliment traditional insect trap surveys in order to better describe the insect community in a given area. Therefore, we compared two methods (a species list of Coleoptera consumed by the birds characterized by metabarcoding and a species list of Coleoptera captured in traditional traps characterized by morphological identification) deployed at the same locations in two elevations to see if metabarcoding bird feces could be used as a viable addition to saproxylic Coleoptera surveys.
Methods
Study area and sample collection
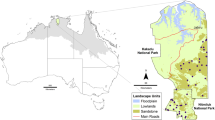
Our study was conducted in Vall d’Ordino, a valley located within three km of Vall de Sorteny Natural Park in the parish of Ordino, Andorra. Five ~ 0.1 km2 plots were selected in black pine (Pinus mugo species complex) forest between 1719 and 2222 masl. In each plot, one or two mist nets and seven traditional insect traps (three attraction, three flight intercept, and one white malaise trap) were deployed.
Percent open space around each trap or mist net (1000 m radius) location was calculated using QGIS3.4 and the MCSA 2012 landcover map downloaded from the Institute of Andorran Studies (Centre de Biodiversitat de l’Institut d’Estudis Andorrans 2012). Plots were characterized as “high” or “low” elevation depending on placement above or below the median elevation of all plots (i.e., 2064 masl).
Attraction traps consisted of a 1 L plastic bottles with a single hole cut in the side, hung upright 30 cm from a live tree trunk. Each bottle was filled with ~ 250 g of a bulk bait mixture of 7 L sangria (Don Simon), 2 L peach juice (Spar), and 1 kg each of salt and sugar (Viñolas et al. 2009). Flight intercept traps consisted of two clear plastic panes perpendicularly crossed below a 14 cm diameter white hard disk attached to a white funnel 13 cm in diameter. Each white malaise trap measured 120 × 100 × 150 cm (Entosphinx S.R.O). A collection bottle containing 70% propylene glycol (VWR Chemicals) and a few drops of dish detergent was attached to each flight intercept and malaise trap. Malaise traps capture higher percentages of Hymenoptera and Diptera (Karlsson et al. 2020) but successfully capture Coleoptera as well (Skvarla and Dowling 2017). All traditional traps were spaced at least 30 m apart. Traps were installed May 23–28, 2017, as mid-May is when snow traditionally recedes from the Andorran tree-line, and their contents were removed and baits refilled every 13–15 days until September 30–31, 2017. All specimens captured in the traditional traps were kept in 70% ethanol until processed.
Mist nets were deployed in the same plots as the traditional traps between May 15, 2018, and September 30, 2018, and consisted of 3 × 6 m and 3 × 9 m long black polyester mesh nets with mesh size of 16 mm2 strung perpendicular to the ground between 2.5 m high poles. Three nets were installed in all sites except one; in this site due to geography only two nets were needed in order to capture the maximum number of birds. Nets were installed for ~ two hours every two weeks depending on weather (i.e., no rain or extreme wind) and on a rotating schedule in order to capture the most birds possible at a given site. Feces from Passerine birds captured in the mist nets were collected with a single use toothpick and stored in molecular grade alcohol on ice and transferred to long-term deep-freeze as soon as possible. A total of 132 fecal samples were collected. All 14 bird species collected are common and eat a wide variety of insects, and a list of bird species collected can be found in Appendix 1.
Species identification and sequencing
Through consulting with group specialists (listed in Table 1), we morphologically identified all Coleoptera specimens from traditional traps to the species level, with the exception of Scydmaeninae. Morphotypes of Staphylinidae were sent to a Staphylinidae expert for species identification. Species were then assigned to functional larval feeding guilds based on the literature currently available regarding each species’ lifecycle as well as the FRISBEE database (Bouget et al. 2008).
DNA from the feces samples (each sample weighed ~ 3 mg) were isolated using QIAamp DNA Stool Mini Kits (QIAGEN) following the manufacture’s protocol with adjustments as suggested by Davies et al. (2022). These adjustments include lowering 25 µl proteinaseK to 20 µl in step four and 600 µl supernatant to 400 µl in step five. Four negative controls (i.e., no sample) were conducted alongside the extraction procedure. DNA concentration was quantified using a Qubit 3.0 Fluorometer (Thermo Fisher Scientific Inc.) and samples were sent to the Georgia Genomics and Bioinformatics Core (University of Georgia, Athens GA, USA) for library preparation and sequencing on the Illumina MiSeq platform (Illumina) using v3 chemistry with 600 cycles of 2 × 250 bp paired-end read lengths. The primer pair ANML (Jusino et al. 2017) was used to amplify a 180 bp segment of the mitochondrial gene, cytochrome oxidase C subunit 1 (COI).
Samples were sequenced together with five mock communities of arthropods created with specimens caught in traditional traps (mock community composition in Appendix 2), as mock communities allow verification of taxonomic coverage and sequencing bias (Braukmann et al. 2019). Each insect chosen for the mock community was identified morphologically to family, and Coleoptera specimens chosen for mock community were morphologically identified to species before subsequent verification by sequencing. All specimens were dipped in a 1% concentration of detergent (Thermo Scientific Tween-80), placed in a sonicating water bath for 60 s, then moved to sterile distilled water. The head, wings and legs of individual specimens were collected, placed in a buffer solution, and macerated with a sterile pestle. DNA was extracted and quantified using the same protocol as for feces. Specimens were taxonomically identified by Sanger sequencing from the amplicons produced with conventional PCR using the LCO1490 and HC02198 primers (Folmer et al. 1994). The PCR was assembled as follows: for a final volume of 25 μl, each reaction contained a final concentration of 0.2 mM dNTP, 2.5 mM MgCl2, 0.4 μm of each primer, 1X buffer, Taq polymerase (Promega) and 1 μl of template DNA. Reactions were run on a Mastercyler Gradient thermocycler (Eppendorf) following the conditions specified in Folmer et al. (1994) and amplicons were visualized in 1% agarose gel. Successfully amplified samples were sent for Sanger sequencing at Eton Bioscience Inc. (Raleigh, NC, USA) and results were queried using the NCBI BLAST algorithm tool (National Center for Biotechnology Information) and BOLD (Barcode of Life Data System) to identify the specimen to the species level. Species identity was assigned if the sequence with the highest percent identity had a value of 96% query cover or above (Jedlicka et al. 2013).
Bioinformatic analysis
The script of the bioinformatic pipeline used in this study can be found in Appendix 3. In brief, the paired-end amplicon fastq files generated from the Illumina MiSeq sequencer were demultiplexed and primers were removed using the open-source bioinformatics pipeline QIIME 2 2020.6 (Bolyen et al. 2019). Lower quality nucleotides on the amplicons were trimmed and truncated, and the amplicons de-noised with DADA2 (via q2-dada2) through a series of filtering, merging of paired reads, and de-replication (Callahan et al. 2016). The end product of the DADA2 pipeline is an ASV (amplicon sequence variant) table, which offers a higher specificity than traditional OTU-level flows (Prodan et al. 2020). An ASV value represents the number of times an error-free unique amplicon sequence variant is recovered from sequencing. The DADA2 pipeline uses error models to divide amplicon reads into partitions, bypassing clustering methods that use fixed dissimilarity methods (Callahan et al. 2016). Potential contaminants in the ASV table were identified by the package Decontam (Davis et al. 2018). Taxonomy was assigned using a classy-sklearn naïve Bayes via q2-feature-classifier approach referencing the “tidybug” database, a training set of arthropod records curated by O’Rourke et al. (2020). Complementing the taxonomy classification based upon the curated database, assignations were checked on a case-by-case basis (see Irion et al. 2020, Smith et al. 2020, Milazzo et al. 2021, and Ratcliffe et al. 2021) and the following protocol was carried out: (1) After taxonomy assignation was complete, assignations were individually checked for locational range. If an ASV was assigned to a species not found in Europe, it was removed. (2) If an ASV was assigned to a rare species or a species that is not currently found in the Pyrenees but is found in Europe, it was flagged. If multiple sequences were assigned to a single flagged species identity, the sequences were aligned to check for sequencing error and the sequences below an 80% sequencing error were removed, leaving a consensus sequence. (3) All flagged assignments were then queried using the NCBI BLAST algorithm tool, and assignments that did not score at or above 98% identity were removed (Brandt et al. 2021; Ritter et al. 2022). (4) Multiple hits showing the same max score on the NCBI BLAST tool were removed from analysis.
Mock community
The species assignments given to the mock community ASVs by metabarcoding were compared with the species assignments of the mock community by sanger sequencing, and the ratio of bias according to taxonomic rank (i.e. the level of uncertainty) was calculated.
Statistical analyses
Mean species richness among plots in high and low elevation and the four collection types were calculated, and the number of Coleoptera species aggregated by family caught by traditional and feces collections were tallied. To compare species richness among collection types, the data was fit to a generalized linear mixed-effects model using the lme4 v.26 package in R (Bates et al. 2015). Open space within a 1 km radius, elevation, and collection type were used as explanatory factors, and plot as random. After the richness model was fit, post-hoc Tukey tests were carried out to investigate error rates of the categorical factor of season and trap type. The Jaccard dissimilarity index was applied to the data using the “vegdist” function of the vegan package and “jaccard” as the method (Oksanen et al. 2020) to examine differences within and among collection types. The above analyses were performed on collection type results that consisted of all Coleoptera species found, and just saproxylic (sensu Speight 1989) species. They were also performed on all the traditional traps combined versus feces collection.
Further examination of differences in species richness between the collection types was conducted by creating sample-size based rarefaction and extrapolation curves of Shannon diversity with 95% confidence intervals using the iNEXT package in R (Chao and Jost 2012; Chao et al. 2014). The iNEXT procedure uses presence/absence data to create a sample-based (in our case traditional and feces collection based) rarefaction curve for the species found in the four collection types, and then estimates the numbers of sample units or percentage of sample coverage present in the assemblage but not represented in the traditional and/or feces collections. Sample coverage can be defined as “the percentage of the total number of individuals in a community that belong to the species represented in the sample” (Chao and Jost 2012).
Finally, the species richness of each larval functional feeding guild (ie. “functional guild richness”) per collection type was calculated. Differences of collection types within a guild were examined using zero-truncated poisson regression with models fit by the glmmTMB package (Brooks et al. 2017) in R (R Core Team 2021), and P values were calculated using Tukey tests. Parasitic and myrmecophilous Coleoptera were considered predators for the purpose of this analysis. Rhizophagous and herbivorous Coleoptera were considered phytophages, and coprophagous, necrophagous, and fungivorous Coleoptera were considered detritivores. Differences in functional guild composition among the collection types were also plotted using the elbow method to define the optimal number of clusters, followed with a k-means cluster analysis (Maechler et al. 2021).
Results
A total of 5506 individual specimens was collected in the traditional traps, representing 36 Coleoptera families, 153 species, and 15 unresolved taxa (interpreted herein as separate species). In the feces samples, 8.95 million sequence reads were produced, identified as the DNA of 19 Coleoptera families, 51 species, and 11 unresolved taxa (interpreted herein as separate species) (Appendix 4). The ANML primer amplified Coleoptera taxa in 74.2% of the samples, and the ASV counts per feces sample ranged from 4 to 126,095 (Supplementary material 2). The comparison of species assignments given to the mock community ASVs by metabarcoding sanger sequencing displayed a 16.67% uncertainty in the assignment to order, and an additional 33.3% uncertainty in the assignment to family.
All Coleoptera species
Collection type was a significant factor in the model, with malaise (mean of 6.35±7.59 species per sample) and flight intercept (mean of 6.35±4.65) containing the highest species richness, followed by feces (2.06±1.18) and attraction (1.52±0.754) (beta-estimates and P values in Table 2) (Fig. 1A). Tukey tests showed richness levels between malaise and flight intercept collection types and between attraction and feces collection types were not significantly different (Table 2). Elevation and percent of open space within a 1 k radius (beta-estimates and P values in Table 2) were not significant.
Saproxylic Coleoptera
As with all Coleoptera captures, collection type (with only saproxylic Coleoptera was taken into consideration) was a significant factor (attraction, mean of 1.32±0.58 species per sample; feces collection, 1.37±0.58; flight intercept, 4.94±3.58; and malaise, 4.2±4.2) (estimates and P values in Table 2) (Fig. 1B). Similar to results of all Coleoptera, richness levels between malaise and flight intercept collection types and between attraction and feces collection types were not significantly different (Table 2). The percent of open land within a 1 k radius and elevation also did not influence richness (beta-estimates and P values in Table 2).
Combined traditional traps of all Coleoptera versus feces collections
Richness levels of traditional trap collections of all Coleoptera were significantly different than feces collections (est.: 0.91, P < 0.001). Elevation and percent of open space within a 1 k radius did not affect species richness (beta-estimates and P-values in Table 3).
Composition dissimilarity
The Jaccard dissimilarity index indicated that the species composition both within and among all collection types were distinctly different. Species composition within attraction traps had the least differences, although composition was still distinctly different (0.87 out of 1). All other trap within/among index results were above 0.92. Similar to the data that includes all Coleoptera, a Jaccard index examination showed very different compositions of saproxylic species within and among all collection types, with a dissimilarity index over 0.78 within attraction traps, and over 0.91 between and among all other collection type combinations. When all traditional collection results were combined into one variable, the Jaccard index continued to measure distinct species differences between and among all traditional collections combined and feces collections.
Twelve Coleoptera species were found in both traditional and feces collections (Appendix 4). Five of these 12 were saproxylic species (Appendix 4). Thirty-nine species were found in just feces collections and 141 were found in just traditional collections (Appendix 4). Of the 141 species found in only traditional collections, 112 are Andorran records, and nine Andorran record species were found in both types of collections (feces and traditional). Records are defined as previously uncollected in Andorra, according to the most recent editions of the Catalogue of Palearctic Coleoptera (Löbl and Smetana 2011, 2013a, b, c; Löbl and Löbl 2015, 2016; Iwan and Löbl 2020). Some of the species found in traditional collections are unusual, such as Axinotarsus tripatriae Constantin 2013, a new species recently described (Constantin 2013) and Curtimorda maculosa (Naezen, 1794), rare in the Iberian Peninsula. Other rare species include Atheta parapicipennis Brundin, 1954 and A. nigritula (Gravenhorst, 1802) and Pityophthorus glabratus Eichhoff, 1878 and Pityogenes conjuntus (Reitter, 1887).
57% of species found only in traditional collections were saproxylic, compared to 36.0% found only in feces collections (Appendix 4). Sixteen saproxylic species were found only in feces and 92 saproxylic species were found only by traditional traps (Appendix 4). Staphylinidae comprised 18.8% of species in traditional traps, versus 14.0% in feces collections (Fig. 2). Curculionidae comprised 12.5% of species found in traditional traps, compared to 24.0% in feces (Fig. 2).
Functional guild analysis: Jaccard dissimilarity, modeling, and k-means cluster analysis
A Jaccard dissimilarity analysis showed distinct differences within and among collection types when species were partitioned into larval functional guilds; all dissimilarity measures were above 0.92. Indeed, collection types caught different functional guilds (Beta estimates and P values in Table 4) (Fig. 3A–D). Flight intercept traps harbored significantly higher richness of predators and wood-feeders than attraction collections (Fig. 3A,C). Richness of phytophagous guilds were significantly higher in malaise than flight intercept and feces collections, and feces collections were moderately higher than flight intercept collections (Fig. 3D). Too few phytophagous guild Coleoptera were found in attraction collections to analyze, and too few detritivore guild Coleoptera were found in bird feces collections to analyze (Beta estimates and P values in Table 4).
A–D Boxplot of species richness (y-axis) per collection type (x-axis) when results are partitioned into larval functional guilds (ie. “functional guild richness”). The four most common larval guilds are shown. Boxes under a common letter are not significantly different according to Tukey tests. A: Attraction, B: Bird feces, F: Flight intercept, M: Malaise *graphics program used to create artwork: R, followed by MS powerpoint
K-means hierarchical clustering results showed attraction and feces collections tended to cluster together when species were organized into functional guilds (Fig. 4). These results are similar to generalized linear model results in which both species richness (see Fig. 1A,B) and functional guild richness (see Fig. 3A–D) of attraction traps and feces trended together at lower richness levels than flight intercept and malaise.
Scatter plot of k-means hierarchical cluster analysis. Points represent each collection type (n=211). Points are clustered according to richness of each larval functional guild per collection type. A: Attraction, B: Bird feces, F: Flight intercept, M: Malaise *graphics program used to create artwork: R
Rarefaction and extrapolation curves
A rarefaction and extrapolation curve created by iNEXT estimated that 160 malaise, 175 attraction, 256 feces, and 417 flight intercept collections would be needed to approach 99% completeness sample coverage (Although 95% confidence intervals show flight intercept and malaise are the only collection types that do not overlap) (Fig. 5A). At 99% sample coverage, feces collections are expected to have higher diversity than attraction traps (29.2 ± 6.4), and lower diversity than malaise and flight intercept traps. 256 feces collections would be needed to reach 99% sample coverage (Fig. 5B).
A, B Sample-size based rarefaction (solid line) and extrapolation (dotted line) curves with 95% confidence intervals for Coleoptera diversity found in four collection types: attraction, feces, flight intercept, and malaise. A Percent of sample coverage (i.e. total probability of occurrence of the species observed in the sample) with respect to number of sample units (collections). B Shannon diversity estimates in rarefied and extrapolated samples with respect to number of sample units (collections). Numbers in parentheses indicate number of sample units necessary to reach 99% coverage, and insect diversity at 99% coverage with 95% confidence intervals. Feces collections are expected to have higher diversity than attraction traps (29.2±6.4), and lower diversity than malaise and flight intercept traps. 256 feces collections would be needed to reach 99% sample coverage. A: Attraction, B: Bird feces, F: Flight intercept, M: Malaise *graphics program used to create artwork: R, followed by MS powerpoint
Discussion
Metabarcoding is a rapidly evolving technology with many untapped potential applications. Our study compared two methods of surveying saproxylic Coleoptera diversity (i.e., metabarcoding birds feces and deploying traditional traps), with the aim of adding metabarcoding bird feces as a viable addition to Coleoptera biodiversity surveys. The results showed (1) the four collection types (metabarcoding and three types of traditional traps) caught different species and different levels of functional guild richness, and (2) metabarcoding of bird feces successfully recorded a segment of taxonomic and functional diversity that both overlapped with and were distinct from traditional trap species lists in our plots. This approach is becoming less expensive but also is limited by disadvantages, including biases that can lead to incorrect data output (O’Rourke et al. 2020).
The collections of the four collection types had very little taxonomic overlap, and rarefaction and extrapolation curves show additional samples of all four collection types would be needed to fulfill the taxonomic diversity potential of each collection type (Fig. 5B). Dissimilarity within all collection types was high. The birds in our study also consumed a wide variety of prey, and high among-feces dissimilarity was also recorded in other dietary metabarcoding studies involving insectivorous Passerines (Shutt et al. 2020) and insectivorous bats (Vesterinen et al. 2016). Thus, the addition of metabarcoding would very likely add additional information to a traditional trap schema, but the results also suggest future studies deploying different collection types should take into account varying levels of collection type efficiency (see Alinvi et al. 2007 and Silva et al. 2018).
Collections of attraction traps and feces tended to cluster together in terms of functional guilds richness per collection type, probably due to their lower levels of species and functional richness when compared to flight intercept and malaise traps (species richness: Fig. 1; functional guild richness: Fig. 3; cluster analysis: Fig. 4). However, the functional richness of phytophage guild Coleoptera in feces collections was moderately higher than flight intercept (Table 4; Fig. 3D), indicating future studies focusing on phytophage Coleoptera would be well served with additional metabarcoding feces surveys. The difference in functional richness caught by fecal metabarcoding is unsurprising as the diets of many Passerine birds (especially during migration and nesting season) depend heavily on phytophagous insects (Tallamy and Shriver 2021). Flight intercept, malaise, and attraction traps are designed to capture mobile arthropods (Yi et al. 2012), while birds can actively search out sessile arthropods and less mobile larval stages of many phytophagous and saproxylic insects. For example, Rhamphus pulicarius (Herbst, 1795), a leaf miner captured in our study only in feces (Appendix 4), has no abdominal legs in larval form (Morris 2012). Another study comparing metabarcoding results of malaise and soil samples found numerous winged dipterans identified in the metabarcoding soil results (likely as eggs or larvae) but not the malaise samples, possibly due to the short flight season of these animals (Kirse et al. 2021).
It is also important to underline the fact that because our study was conducted from spring to fall (overlapping migration and nesting seasons), our dietary results could be very different than a Passerine dietary study conducted in winter; many resident omnivorous Passerine birds transition to a more herbivorous diet in the winter (Chamberlain et al. 2007; Renner et al. 2012). Furthermore, otherwise sedentary Passerines, such as the crested tit [Lophophanes cristatus (L., 1758)], can make short migration movements in especially harsh winters (Busse 1995). Examining the differences in diet between species of Passerine birds, however, is out of the scope of this paper, as the focus herein was to capture the most birds possible in a given site in order to explore the ways in which feces collections by mist netting could complement traditional insect traps.
In our plots, metabarcoding of bird feces successfully recorded a segment of taxonomic and functional diversity distinct from traditional trap species lists. The high level of species richness recorded in the birds’ feces is supported by other fecal metabarcoding studies involving insectivorous and/or omnivorous Passerines (Ribeiro et al. 2019; Rytkönen et al. 2019; Shutt et al. 2020; Silva et al. 2020). However, there was also significant overlap in our study; 12 species were shared between traditional and feces collections (9 of the 12 were country records). This proves the value of incorporating metabarcoding bird feces to document diversity as opposed to relying only on traditional traps, especially for projects designed to detect the presence of a rare or specific insect species or the presence of a forest pest. A database composed of DNA extracted from voucher specimens of a species of interest, with a mock community that also includes this extracted DNA, would allow for a focused study plan with fewer biases inherent in the metabarcoding pipeline. Furthermore, as metabarcoding becomes more widely used and less expensive, family or group experts would be unnecessary.
Nonetheless, there still exists certain disadvantages to dietary metabarcoding. Metabarcoding relies on characterizing DNA sequences. Transforming these sequence counts into individual abundance (the number of individual specimens per taxa in the feces sample) is thus far unattainable (Piper et al. 2019). Capturing relative abundance (the percentage of a taxa within a feces sample) is difficult due to technological and biological biases such as differential PCR amplification and DNA extraction efficiency (Deagle et al. 2013, 2019; Piñol et al. 2018). Most metabarcoding studies including ours transform sequence counts into a presence/absence matrix (O’Rourke et al. 2020). However, PCR-free approaches show promise in attaining accurate abundance measures in biological assessments using metagenomics (Liu et al. 2016). As metabarcoding is a novel and rapidly evolving technology, the rate of false positive errors can also be high due to potential biases including inappropriate filtering thresholds and chimeric fragments (Zinger et al. 2019). Furthermore, insects are still poorly represented in the NCBI reference databases (Schoch et al. 2020), which highlights the need for more comprehensive and curated databases. A complete reference database is especially important as other fecal adult Passerine metabarcoding studies have recorded surprisingly high species rich diets (Shutt et al. 2020). The dearth of insect DNA in reference databases can lead to taxonomic classification errors or record gaps (Geiger et al. 2016). The list of species from our metabarcoding study, for example, had high levels of uncertainty; for Coleoptera, the mock community showed a 33.3% uncertainty in the assignment of species, 33.3% uncertainty in the assignment of order, and 16.7% uncertainty in the assignment of class. Finally, the influence of digestion must be a consideration when regarding the recovery and detection of fecal DNA, as both physical and chemical processes will govern unequal DNA processing (Snider et al. 2021).
Parmain et al. (2013) reported a 20% mean assemblage dissimilarity between years in saproxylic Coleoptera. We acknowledge that our comparisons between our collecting methods could have been affected by normal population fluctuations. In our study, logistical reasons resulted in traditional sampling and feces sampling being conducted in subsequent years. However, the magnitude of difference in species richness and functional guilds observed in the traditional traps compared to that found in the feces suggests these differences should not be solely due to annual variation. To our knowledge, our study is the first to compare adult Passerine bird feces to collections of traditional insect traps using metabarcoding. However, another recent study compared Passerine nestling feces to insect frass collected in different years and found the technique to have promising applications (Rytkönen et al. 2019).
Even with these limitations (abundance count issues, false positive or negative errors, digestion considerations, and database restraints), the ability to monitor Coleoptera populations while simultaneously performing a survey of the bird population highlights the potential usefulness of metabarcoding technology. Our study compared Coleoptera species lists obtained using morphological identification from insects collected from traditional insect traps and metabarcoding procedures performed on birds’ feces and found distinct compositions of taxonomic and functional diversity. Numerous species, including several country records, were found in both collections. Upscaling taxa classification in bulk samples (such as feces collections) that would otherwise be impossible due to time and cost constraints, could be an effective, albeit unusual, application for this rapidly evolving technology.
References
Alinvi O, Ball JP, Danell K, Hjalten J, Pettersson RB (2007) Sampling saproxylic beetle assemblages in dead wood logs: comparing window and eclector traps to traditional bark sieving and a refinement. J Insect Conserv 11:99–112
Andrews KR, Gerritsen A, Rashed A, Crowder DW, Rondon SI, van Herk WG, Vernon R, Wanner KW, Wilson CM, New DD, Fagnan MW, Hohenlohe PA, Hunter SS (2020) Wireworm (Coleoptera: Elateridae) genomic analysis reveals putative cryptic species, population structure, and adaptation to pest control. Commun Biol 3:489
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Bolyen E et al (2019) Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 37:852–857
Bouget C, Brustel H, Zagatti P (2008) The FRench Information System on Saproxylic BEetle Ecology (FRISBEE): an ecological and taxonomical database to help with the assessment of forest conservation status. Rev Ecol 10
Brandt MI, Trouche B, Quintric L, Günther B, Wincker P, Poulain J, Arnaud-Haond S (2021) Bioinformatic pipelines combining denoising and clustering tools allow for more comprehensive prokaryotic and eukaryotic metabarcoding. Mol Ecol Resources 21:1904–1921
Braukmann TWA, Ivanova NV, Prosser SWJ, Elbrecht V, Steinke D, Ratnasingham S, de Waard JR, Sones JE, Zakharov EV, Hebert PDN (2019) Metabarcoding a diverse arthropod mock community. Mol Ecol Resour 19:711–727
Brooks ME, Kristensen K, van Benthem KJ, Magnusson A, Berg CW, Nielsen A, Skaug HJ, Maechler M, Bolker B (2017) glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J 9:378–400
Busse P (1995) The migratory movements of the Crested Tit (Parus cristatus) a monitoring aspect. Ring 17:38–45
Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP (2016) DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods 13:581–583
Centre de Biodiversitat de l’Institut d’Estudis Andorrans (2012) Sistema d’Informació Geogràfica i Mediambiental d’Andorra. https://www.iea.ad/mapa-de-cobertes-del-sol-d-andorra-2012 [Accessed May 12, 2021]
Chamberlain DE, Gosler AG, Glue DE (2007) Effects of the winter beechmast crop on bird occurrence in british gardens. Bird Study 54:120–126
Chao A, Jost L (2012) Coverage-based rarefaction and extrapolation: standardizing samples by completeness rather than size. Ecol 93:2533–2547
Chao A, Gotelli NJ, Hsieh TC, Sander EL, Ma KH, Colwell RK, Ellison AM (2014) Rarefaction and extrapolation with Hill numbers: a framework for sampling and estimation in species diversity studies. Ecol Monogr 84:45–67
Constantin R (2013) Contribution à la connaissance des Malachiidae d’Europe et description de deux espèces nouvelles (Coleoptera, Cleroidea). Bull Soc Entomol Fr 118:305–320
Crisol-Martínez E, Moreno-Moyano LT, Wormington KR, Brown PH, Stanley D (2016) Using next-generation sequencing to contrast the diet and explore pest-reduction services of sympatric bird species in macadamia orchards in Australia. PLoS ONE 11:e0150159
Davies SR, Vaughan I, Thomas R, Marchbank A, Drake L, Symondson WOC (2022) Seasonal and ontological variation in diet and age-related differences in prey choice, by an insectivorous songbird. Authorea. https://doi.org/10.1002/ece3.9180
Davis NM, Proctor DM, Holmes SP, Relman DA, Callahan BJ (2018) Simple statistical identification and removal of contaminant sequences in marker-gene and metagenomics data. Microbiome 6:226
Deagle BE, Thomas AC, Shaffer AK, Trites AW, Jarman SN (2013) Quantifying sequence proportions in a DNA-based diet study using Ion Torrent amplicon sequencing: which counts count? Mol Ecol Resour 13:620–633
Deagle BE, Thomas AC, McInnes JC, Clarke LJ, Vesterinen EJ, Clare EL, Kartzinel TR, Eveson JP (2019) Counting with DNA in metabarcoding studies: how should we convert sequence reads to dietary data? Mol Ecol 28:391–406
DeSalle R, Egan MG, Siddall M (2005) The unholy trinity: taxonomy, species delimitation and DNA barcoding. Philos Trans Biol Sci 360:1905–1916
Dirzo R, Young HS, Galetti M, Ceballos G, Isaac NJ, Collen B (2014) Defaunation in the Anthropocene. Sci 345(6195):401–406
Folmer O, Black M, Hoeh W, Lutz R, R V (1994) DNA primers for amplification of mitochondrial cytochrome C oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol 3:294–299
Geiger MF, Moriniere J, Hausmann A, Haszprunar G, Wägele W, Hebert PDN, Rulik B (2016) Testing the global malaise trap program – how well does the current barcode reference library identify flying insects in Germany? Biodivers Data J 4:e10671
Gimmel M, Ferro M (2018) General Overview of Saproxylic Coleoptera. In: Ulyshen M (ed) Saproxylic insects. Springer, Cham, Switzerland, pp 51–128
Hajibabaei M, Porter TM, Wright M, Rudar J (2019) COI metabarcoding primer choice affects richness and recovery of indicator taxa in freshwater systems. PLoS ONE 14:e0220953
Hallmann CA, Sorg M, Jongejans E, Siepel H, Hofland N, Schwan H, Stenmans W, Müller A, Sumser H, Hörren T, Goulson D, de Kroon H (2017) More than 75% decline over 27 years in total flying insect biomass in protected areas. PLoS ONE 12:e0185809
Hardersen S, Zapponi L (2017) Wood degradation and the role of saproxylic insects for lignoforms. Appl Soil Ecol. 123:334–338
Hardulak LA, Moriniere J, Hausmann A, Hendrich L, Schmidt S, Doczkal D, Muller J, Hebert PDN, Haszprunar G (2020) DNA metabarcoding for biodiversity monitoring in a national park: screening for invasive and pest species. Mol Ecol Resour 20:1542–1557
Irion S, Jardillier L, Sassenhagen I, Christaki U (2020) Marked spatiotemporal variations in small phytoplankton structure in contrasted waters of the Southern Ocean (Kerguelen area). Limnol Oceanogr 65:2835–2852
Iwan D, Löbl I (eds) (2020) Tenebrionoidea. Catalogue of Palaearctic Coleoptera. Koninklijke Brill NV, Leiden, The Netherlands
Jedlicka JA, Sharma AM, Almeida RPP (2013) Molecular tools reveal diets of insectivorous birds from predator fecal matter. Conserv Genet Resour 5:879–885
Jedlicka JA, Vo A, Almeida RP (2017) Molecular scatology and high-throughput sequencing reveal predominately herbivorous insects in the diets of adult and nestling western bluebirds (Sialia mexicana) in California vineyards. Auk 134:116–127
Jusino MA, Banik MT, Palmer JM, Wray AK, Xiao L, Pelton E, Barber JR, Kawahara AY, Gratton C, Peery MZ, Lindner DL (2017) An improved method for utilizing high-throughput amplicon sequencing to determine the diets of insectivorous animals. Mol Ecol Resour 19:176–190
Kamiński MJ, Lumen R, Kubicz M, Steiner W, Kanda K, Iwan D (2019) Immature stages of beetles representing the ‘Opatrinoid’ clade (Coleoptera: Tenebrionidae): an overview of current knowledge of the larval morphology and some resulting taxonomic notes on Blapstinina. Zoomorphology 138:349–370
Karlsson D, Hartop E, Forshage M, Jaschhof M, Ronquist F (2020) The swedish malaise trap project: a 15 year retrospective on a countrywide insect inventory. Biodivers Data J 8:e47255
Karpiński L, Maák I, Wegierek P (2021) The role of nature reserves in preserving saproxylic biodiversity: using longhorn beetles (Coleoptera: Cerambycidae) as bioindicators. Eur Zool J 88:487–504
Kirse A, Bourlat SJ, Langen K, Fonseca VG (2021) Metabarcoding malaise traps and soil eDNA reveals seasonal and local arthropod diversity shifts. Sci Rep 11:10498–10498
Liu S, Wang X, Xie L, Tan M, Li Z, Su X, Zhang H, Misof B, Kjer KM, Tang M, Niehuis O, Jiang H, Zhou X (2016) Mitochondrial capture enriches mito-DNA 100 fold, enabling PCR-free mitogenomics biodiversity analysis. Mol Ecol Resour 16:470–479
Liu M, Clarke LJ, Baker SC, Jordan GJ, Burridge CP (2020) A practical guide to DNA metabarcoding for entomological ecologists. Ecol Entomol 45:373–385
Löbl I, Löbl D (eds) (2015) Hydrophiloidea - Staphylinoidea (2 vols): revised and updated Edition. Catalogue of Palaearctic Coleoptera, 2nd edn. Koninklijke Brill NV, Leiden, The Netherlands
Löbl I, Löbl D (eds) (2016) Scarabaeoidea – Scirtoidea – Dascilloidea – Buprestoidea – Byrrhoidea. Catalogue of Palaearctic Coleoptera. Koninklijke Brill NV, Leiden, The Netherlands
Löbl I, Smetana A (eds) (2011) Curculionoidea I. Catalogue of Palaearctic Coleoptera. Apollo Books, Stenstrup, Denmark
Löbl I, Smetana A (eds) (2013a) Curculionoidea II. Catalogue of Palaearctic Coleoptera. Koninklijke Brill NV, Leiden, The Netherlands
Löbl I, Smetana A (eds) (2013b) Chrysomeloidea. Catalogue of Palaearctic Coleoptera. Koninklijke Brill NV, Leiden, The Netherlands
Löbl I, Smetana A (eds) (2013c) Elateroidea, Derodontoidea, Bostrichoidea, Lymexyloidea, Cleroidea and Cucujoidea. Catalogue of Palaearctic Coleoptera. Koninklijke Brill NV, Leiden, The Netherlands
Losey JE, Vaughan M (2006) The economic value of ecological services provided by insects. Biosci 56:311–323
Macfadyen S, Moradi-Vajargah M, Umina P, Hoffmann A, Nash M, Holloway J, Severtson D, Hill M, Van Helden M, Barton M (2019) Identifying critical research gaps that limit control options for invertebrate pests in australian grain production systems. Austral Entom 58:9–26
Maechler M, Rousseeuw P, Struyf A, Hubert M, Hornik K (2021) cluster: Cluster Analysis Basics and Extensions, Version 2.1.1
Micó E, Juárez M, Sánchez A, Galante E (2011) Action of the saproxylic scarab larva Cetonia aurataeformis (Coleoptera: Scarabaeoidea: Cetoniidae) on woody substrates. J Nat Hist 45:2527–2542
Milazzo C, Zulak KG, Muria-Gonzalez MJ, Jones D, Power M, Bransgrove K, Bunce M, Lopez-Ruiz FJ (2021) High-throughput metabarcoding characterizes fungal endophyte diversity in the Phyllosphere of a Barley Crop. Phytobiomes J 5:316–325
Milberg P, Bergman K-O, Johansson H, Jansson N (2014) Low host-tree preferences among saproxylic beetles: a comparison of four deciduous species. Insect Conserve Divers 7:508–522
Møller AP (2020) Quantifying rapidly declining abundance of insects in Europe using a paired experimental design. Ecol Evol 10:2446–2451
Morris MG (2012) True weevils (part III) (Coleoptera: Curculioninae, Baridinae, Orobitidinae). RES Handbooks for the identification of british insects Vol. 5 part 17d. Royal Entomological Society, St Albans, England
Nieto A, Alexander KNA (2010) European Red List of Saproxylic Beetles. Publications Office of the European Union. https://doi.org/10.2779/84561
O’Rourke DR, Bokulich NA, Jusino MA, MacManes MD, Foster JT (2020) A total crapshoot? Evaluating bioinformatic decisions in animal diet metabarcoding analyses. Ecol Evol 10(18):9721–9739
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Szoecs E, Wagner H (2020) vegan: Community Ecology Package, Version R package version 2.5-7
Parmain G, Dufrêne M, Brin A, Bouget C (2013) Influence of sampling effort on saproxylic beetle diversity assessment: implications for insect monitoring studies in european temperate forests. Agric For Entomol 15:135–145
Pedlar J, McKenney D, Yemshanov D, Hope E (2019) Potential economic impacts of the asian longhorned beetle (Coleoptera: Cerambycidae) in eastern Canada. J Econ Entomol 113(2):839–850
Piñol J, Senar MA, Symondson WOC (2018) The choice of universal primers and the characteristics of the species mixture determine when DNA metabarcoding can be quantitative. Mol Ecol 28:407–419
Piper AM, Batovska J, Cogan NOI, Weiss J, Cunningham JP, Rodoni BC, Blacket MJ (2019) Prospects and challenges of implementing DNA metabarcoding for high-throughput insect surveillance. Gigascience 8:22
Porter TM, Morris DM, Basiliko N, Hajibabaei M, Doucet D, Bowman S, Emilson EJS, Emilson CE, Chartrand D, Wainio-Keizer K, Séguin A, Venier L (2019) Variations in terrestrial arthropod DNA metabarcoding methods recovers robust beta diversity but variable richness and site indicators. Sci Rep 9:18218
Prodan A, Tremaroli V, Brolin H, Zwinderman AH, Nieuwdorp M, Levin E (2020) Comparing bioinformatic pipelines for microbial 16S rRNA amplicon sequencing. PLoS ONE 15:e0227434
R Core Team (2021) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Version. R Core Team, Vienna, Austria
Ratcliffe FC, Uren Webster TM, Rodriguez-Barreto D, O’Rorke R, Garcia de Leaniz C, Consuegra S (2021) Quantitative assessment of fish larvae community composition in spawning areas using metabarcoding of bulk samples. Ecol Appl 31:e02284
Renner SC, Baur S, Possler A, Winkler J, Kalko EKV, Bates PJJ, Mello MAR (2012) Food preferences of winter bird communities in different forest types. PLoS ONE 7:e53121–e53121
Ribeiro Â, Smit B, Gilbert M (2019) 31° South: dietary niche of an arid-zone endemic passerine. Environ DNA 1:1–10
Ritter CD, Dal Pont G, Stica PV, Horodesky A, Cozer N, Netto OSM, Henn C, Ostrensky A, Pie MR (2022) Wanted not, wasted not: searching for non-target taxa in environmental DNA metabarcoding by-catch. Environ Adv 7:100169
Rytkönen S, Vesterinen EJ, Westerduin C, Leviäkangas T, Vatka E, Mutanen M, Välimäki P, Hukkanen M, Suokas M, Orell M (2019) From feces to data: a metabarcoding method for analyzing consumed and available prey in a bird-insect food web. Ecol Evol 9:631–639
Saint-Germain M, Buddle C, Drapeau P (2006) Sampling saproxylic Coleoptera: scale issues and the importance of behavior. Env Entomol 35:478–487
Sánchez-Bayo F, Wyckhuys KAG (2019) Worldwide decline of the entomofauna: a review of its drivers. Biol Conserv 232:8–27
Schoch CL, Ciufo S, Domrachev M, Hotton CL, Kannan S, Khovanskaya R, Leipe D, McVeigh R, O’Neill K, Robbertse B, Sharma S, Soussov V, Sullivan JP, Sun L, Turner S, Karsch-Mizrachi I (2020) NCBI Taxonomy: a comprehensive update on curation, resources and tools. Database. https://doi.org/10.1093/database/baaa062
Shokralla S, Spall J, Gibson J, Hajibabaei M (2012) Next-generation sequencing technologies for environmental DNA research. Mol Ecol 21:1794–1805
Shutt JD, Nicholls JA, Trivedi UH, Burgess MD, Stone GN, Hadfield JD, Phillimore AB (2020) Gradients in richness and turnover of a forest passerine’s diet prior to breeding: a mixed model approach applied to faecal metabarcoding data. Mol Ecol 29:1199–1213
Shutt JD, Trivedi UH, Nicholls JA (2021) Faecal metabarcoding reveals pervasive long-distance impacts of garden bird feeding. Ecol Environ & Conserv 288:20210480
Silva D, Salamanca J, Kyryczenko-Roth V, Alborn HT, Rodriguez-Saona C (2018) Comparison of trap types, placement, and colors for monitoring Anthonomus musculus (Coleoptera: Curculionidae) adults in highbush blueberries. J Insect Sci 18(2):19
Silva LP, Mata VA, Lopes PB, Lopes RJ, Beja P (2020) High-resolution multi‐marker DNA metabarcoding reveals sexual dietary differentiation in a bird with minor dimorphism. Ecol Evol 10:10364–10373
Skvarla MJ, Dowling APG (2017) A comparison of trapping techniques (Coleoptera: Carabidae, Buprestidae, Cerambycidae, and Curculionoidea excluding Scolytinae). J Insect Sci 17(1):7
Smith PE, Waters SM, Gómez Expósito R, Smidt H, Carberry CA, McCabe MS (2020) Synthetic wequencing standards: a guide to database choice for rumen microbiota amplicon sequencing analysis. Front Microbiol 11:606825
Snider AM, Bonisoli-Alquati A, Pérez-Umphrey AA, Stouffer PC, Taylor SS (2021) Metabarcoding of stomach contents and fecal samples provide similar insights about Seaside Sparrow diet. Ornithol Appl 124:1–12
Sommerfeld A, Rammer W, Heurich M, Hilmers T, Müller J, Seidl R, Matlack G (2021) Do bark beetle outbreaks amplify or dampen future bark beetle disturbances in Central Europe? J Ecol 109:737–749
Speight MCD (1989) Saproxylic Invertebrates and Their Conservation. Council of Europe. 92-871-1679-2
Staniec B, Sałapa D, Pietrykowska-Tudruj E (2014) Comparative morphology of the larvae of the rove beetles of Paederus, Lathrobium, and Tetartopeus, with notes on its systematic position (Coleoptera: Staphylinidae: Paederinae). J Insect Sci 14:190
Stokland JN, Siitonen J, Jonsson BG (2012) The Saproxylic Food web. Biodiversity in Dead Wood. Cambridge Univ Press, Cambridge
Storka NE, McBrooma J, Gelyb C, Hamilton AJ (2015) New approaches narrow global species estimates for beetles, insects, and terrestrial arthropods. PNAS 112:7519–7523
Strid Y, Schroeder M, Lindahl B, Ihrmark K, Stenlid J (2014) Bark beetles have a decisive impact on fungal communities in Norway spruce stem sections. Fungal Ecol 7:47–58
Takano S-I, Mochizuki A, Konishi K, Takasu K, Alouw JC, Pandin DS, Nakamura S (2011) Two cryptic species in Brontispa longissima (Coleoptera: Chrysomelidae): evidence from mitochondrial DNA analysis and crosses between the two nominal species. Annal Entomol Soc Am 104:121–131
Tallamy DW, Shriver WG (2021) Are declines in insects and insectivorous birds related? Ornithol Appl 123:8
Vesterinen EJ, Ruokolainen L, Wahlberg N, Peña C, Roslin T, Laine VN, Vasko V, Sääksjärvi IE, Norrdahl K, Lilley TM (2016) What you need is what you eat? Prey selection by the bat Myotis daubentonii. Mol Ecol 25:1581–1594
Viñolas A, Bentanachs J, Masó G (2009) Biodiversitat de coleòpters en el Parc Natural de Cadí-Moixeró. In: Museu de Ciències Naturals (Zoologia), Barcelona
Wagner DL, Grames EM, Forister ML, Berenbaum MR, Stopak D (2021) Insect decline in the Anthropocene: death by a thousand cuts. Proc Nat Acad Sci 118:e2023989118
West KM, Richards ZT, Harvey ES, Susac R, Grealy A, Bunce M (2020) Under the karst: detecting hidden subterranean assemblages using eDNA metabarcoding in the caves of Christmas Island, Australia. Sci Rep 10:21479
Yi Z, Jinchao F, Dayuan X, Weiguo S, Axmacher JC (2012) A comparison of terrestrial arthropod sampling methods. J Resour Ecol 3:174–182
Yu DW, Ji Y, Emerson BC, Wang X, Ye C, Yang C, Ding Z (2012) Biodiversity soup: metabarcoding of arthropods for rapid biodiversity assessment and biomonitoring. Method Ecol Evol 3:613–623
Zhang Z-Q, Hooper J, van Soest R, Pisera A, Crowther A, Tyler S, Schilling S, Eschmeyer W, Fong J, Blackburn D, Wake D, Wilson D, Reeder D, Fritz U, Hodda M, Guidetti R, Bertolani R, Mayer G, De Sena Oliveira I, Richardson D (2011) Animal biodiversity: an outline of higher-level classification and taxonomic richness. Zootaxa 3148:7–237
Zinger L, Bonin A, Alsos IG, Bálint M, Bik H, Boyer F, Chariton AA, Creer S, Coissac E, Deagle BE, De Barba M, Dickie IA, Dumbrell AJ, Ficetola GF, Fierer N, Fumagalli L, Gilbert MTP, Jarman S, Jumpponen A, Kauserud H, Orlando L, Pansu J, Pawlowski J, Tedersoo L, Thomsen PF, Willerslev E, Taberlet P (2019) DNA metabarcoding—need for robust experimental designs to draw sound ecological conclusions. Mol Ecol 28:1857
Acknowledgements
Josep Muñoz Batet and Amador Viñolas gave invaluable assistance with family identification and insect trap guidance. Gianfranco Liberti, Miguel Alonso-Zarazaga, Eduard Petitpierre Vall donated many hours of instruction regarding the groups Dasytinae, Scolytinae, and Chrysomelidae, respectively. Jorge Mederos was integral to trap design and installation. Jana Marco, Gerald and Barbara Dinkins, Georgia Hawkins, Brent Bookwalter, and volunteers with the Earthwatch Institute provided hours of effort for insect and feces collection and cleaning. Toni Carrasco, Marc Vilella, Xavi Mendez Camps, Pablo Fernandez, Jasmine Leather, Claudia Pla-Narbona, and especially David Hernández provided instrumental effort to sample sorting. Cristina Ametller Quero was extremely helpful with organizing and filtering DNA sequences. Amie Carlone gave valuable GIS advice. Cesc Murria was especially helpful in regard to bioinformatic processes. Roberto Molowny gave very helpful statistical advice. This work was supported by the Earthwatch Institute, the Daniel B. Warnell School of Forestry and Natural Resources within the University of Georgia, and the Collections Section of the Museu de Ciències Naturals de Barcelona.
Funding
Open Access Funding provided by Universitat Autonoma de Barcelona.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bookwalter, J., Niyas, A.M.M., Caballero-López, B. et al. Fecal matters: implementing classical Coleoptera species lists with metabarcoding data from passerine bird feces. J Insect Conserv 27, 557–569 (2023). https://doi.org/10.1007/s10841-023-00479-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-023-00479-7