Abstract
Habitat requirements of the marsh fritillary Euphydryas aurinia have been studied intensively in recent years in order to inform conservation action on this rapidly declining species. Autecological studies have been undertaken on various spatial scales to account for differences in mobility between life stages. It has become clear that resource utilisation by the species varies between different geographic areas and also on a local scale. We studied the microhabitat surrounding Succisa pratensis plants chosen for oviposition and around resulting larval webs in a key Welsh population, UK. Microhabitat preferences were shown to differ between ovipositing females and pre-diapause larval groups. For oviposition, females showed a preference for host plants within clusters of S. pratensis surrounded by a relatively low sward. In contrast, larval webs were found in relatively high vegetation with relatively low S. pratensis cover in the immediate vicinity. The marked difference between oviposition and larval web microhabitat was achieved through very long distance dispersals undertaken by the pre-diapause larval groups. This gives rise to questioning the common assumption that pre-diapause larval web location can be used as a proxy for oviposition location. It also underpins the importance of managing E. aurinia habitats for maximum structural variability on a small scale.
Similar content being viewed by others
Introduction
Many butterfly species are in decline across the globe. In Britain, latest analyses of long-term monitoring data indicate that 70% of species have declined in occurrence and 57% in abundance since 1976 (Fox et al. 2015). The marsh fritillary (Euphydryas aurinia) is a threatened butterfly species, listed in Annex II of the European Community Habitats and Species Directive (92/43/EEC). It is also classified as a priority species under Section 7 of the Environment (Wales) Act 2016 and for England in Section 41 of the Natural Environment and Rural Communities Act 2006 because the UK is one of the remaining strongholds of E. aurinia (van Swaay and Warren 1999). The main threats to E. aurinia are agricultural improvement and changes in agricultural practice, land abandonment and habitat fragmentation (Fowles and Smith 2006; Fox et al. 2006).
E. aurinia is known to occur in metapopulations with periodic extinction and re-colonisation events and it is well established that the successful protection and restoration of resilient populations requires a landscape scale approach, i.e. the consideration of a network of suitable habitat patches within the species’ dispersal ability (Schtickzelle et al. 2005; Bulman 2001; Bulman et al. 2007; Fowles 2011; Ellis et al. 2012). Generally speaking, correlation between host plant abundance and butterfly abundance would be expected to be highest in monophagous, univoltine species of relatively low mobility such as E. aurinia (Dennis et al. 2004). However, it has also been widely recognised that conservation action needs to take into account all life stages of the species, and that habitat quality is determined by resource availability and habitat structure on various scales as defined by the mobility of different life stages (Anthes et al. 2003; Early 2006). It has been argued that habitat quality management for non-adult life stages is of even greater importance than creating suitable habitat for adults because of the more specific habitat requirements of larvae in relation to adults (Smee et al. 2011) and because of the temporal dominance of juvenile over adult stages (García-Barros and Fartmann 2009). In E. aurinia, the adult is only on the wing for a few weeks while larval stages are present for a good 9 months of the year. Thomas et al. (2011) found that larval habitat quality is the primary determinant of habitat carrying capacity for butterflies, stressing the vital importance of larval habitat creation for successful conservation management.
Whilst the patch scale and inter-patch connectivity is important to the mobile adult butterfly and essential for the maintenance of functioning metapopulations, pre-diapause larvae have been reported to be quite sedentary (e.g. Anthes et al. 2003; Early 2006; Tjørnløv et al. 2015). The oviposition site has a direct impact on hatching success, larval performance, and, in the long run, parental fitness (Resetarits 1996). Food availability immediately around the oviposition site is of particular importance for species laying large batches of eggs, in contrast to those laying single eggs (Eichel and Fartmann 2008) and it has been shown to be an important factor in oviposition choice by E. aurinia. In some populations this appears as a preference for larger host plants (Anthes et al. 2003; Liu et al. 2006), in others as a preference for dense groups of host plants (Konvička et al. 2003; Betzholtz et al. 2007; Smee et al. 2011).
Microclimatic conditions at the oviposition site and physical and chemical characteristics of the host plant also appear to influence the female’s decision; Anthes et al. (2003) for example found a preference for host plants with full exposure to the sun. Various studies found that surrounding sward height is important as well, although it appears that optimum sward heights for oviposition and pre-diapause larval development vary between different geographic areas and habitat types. A general preference for shorter swards has been found in cooler climates such as Scotland and Sweden (Ravenscroft and Gaywood 1996; Betzholtz et al. 2007) and also in the chalk grasslands utilised by E. aurinia in parts of southern England (BUTT 1986). The second main habitat for E. aurinia in Britain is in marshy or heathy grasslands such as purple moor grass-rush pastures (Warren 1994). Based on studies of this type of habitat in the south of England, a target average sward height of 12–25 cm has been suggested (Hobson et al. 2002) and this was adapted as the target sward range for ‘Good Condition’ habitat as used in habitat condition mapping in Wales (Fowles and Smith 2006). However, it has not been validated in the field whether the micro-habitats selected by the female for oviposition correspond with ‘Good Condition’ habitat.
The aim of this study was to establish microhabitat preferences of one of the key populations of E. aurinia in Wales for both oviposition and pre-diapause larval development. This was achieved by locating and studying oviposition locations and resulting larval webs to explore small-scale habitat requirements.
Materials and methods
Study species
Euphydryas aurinia (Lepidoptera: Nymphalidae) is a univoltine butterfly. Adults usually emerge between mid-May and early July in Britain. The sole host plant utilised in Wales is Succisa pratensis. The female butterfly emerges carrying a fully developed egg batch (Porter and Ellis 2011). Once mating is completed, the female selects a suitable host plant on which it lays an egg batch of up to 500, but usually no more than 350, eggs on the underside of a leaf (Porter 1981). Subsequent, smaller batches may be laid (Porter 1992). Larvae hatch after 30–40 days and immediately spin a feeding web (Porter 1981). They feed gregariously until the fourth instar larvae create a dense hibernaculum web in September, often at the base of a tussock (Porter 1981, 1983). During the pre-diapause period, the larval groups remain largely coherent, either extending or abandoning and re-building webs in accordance with their increasing feeding requirements (Porter 1981, 1982). After hibernation they split into smaller groups until they are solitary by mid-April (Porter 1982). By early May, the larvae reach the sixth instar and pupate; this usually takes place close to the ground, often suspended from a leaf or stem concealed in low vegetation (Porter 1981).
Study site
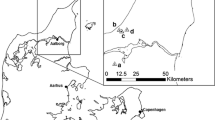
The research was carried out at Rhos Llawrcwrt, a designated site of special scientific interest (SSSI), national nature reserve (NNR) and Special Area of Conservation (SAC) located at around 200 m a.s.l. in Ceredigion (Wales, UK). The size of the NNR is approximately 65.8 ha. The population of E. aurinia has been monitored systematically since the 1980s, based on a network of 53 permanent transects of 50 × 2 m. The population is of national importance and thought to comprise an estimated 10,000 adults in peak years (CCW 2008).
The habitat utilised by E. aurinia at the study site is a mix of purple moor grass-rush pasture and purple moor grass-meadow thistle mire communities and as such representative of the habitats utilised in Wales and much of England (Warren 1994). These habitats are transient and depend on extensive management to be sustained. At the study site, this is achieved by extensive cattle grazing at target stocking rates of 0.3–0.5 livestock units/ha/annum. Cattle and/or ponies are recommended grazing animals for unimproved lowland grassland which supports E. aurinia habitat in Britain (Butterfly Conservation 2004). It has been suggested that optimum conditions for oviposition exist in recently abandoned meadows as well as along the edges of currently grazed meadows (Anthes et al. 2003; Saarinen et al. 2005). For this reason, a ‘rotational exclosure’ trial has been established on site. From autumn 2011 to June 2013, a parcel of the core area utilised by E. aurinia was fenced off to exclude grazing. In June 2013, the fence was moved in order to bring the formerly enclosed area back into grazing and to exclude an adjacent parcel of land from grazing for the 2013/14 grazing season.
Microhabitat for oviposition
Between the 8th and 25th of June 2013, egg batches were located by searching all S. pratensis plants along 38 transects of 50 m length and 2 m width; 32 of these were part of the established infrastructure for E. aurinia monitoring on the reserve and the remaining six were added in to focus the search effort on parts of the site with high E. aurinia activity. One sampling point was established at each host plant that supported egg batches, regardless of the number of egg clusters found on the plant. This was done since several egg batches on the same plant are not necessarily the result of independent decisions as ovipositing females may be attracted by conspecific eggs (Stefanescu et al. 2006). Clutch size was not accounted for since the presence of eggs on a plant, regardless of their number, indicates the acceptance of the plant and its surrounding micro-habitat by the ovipositing female(s). For each sampling point, 10-digit GPS coordinates (UK National Grid) were recorded and the location was marked with a cane.
A null model was established by pairing each of the oviposition plants with a control plant which had not been selected for oviposition. Control plants were determined in early July (at the end of the flight period) by walking three steps in a random direction from each oviposition plant and dropping a 50 cm quadrat. The S. pratensis plant nearest to the central sub-square was checked for egg batches and, if negative, accepted as a control plant and also marked with a cane and recorded on GPS.
In early July, a range of microhabitat parameters was recorded around each of the oviposition and control plants. First, vegetation height was measured as close as possible to each sample plant with a drop disc (Stewart et al. 2001). A 50 cm quadrat subdivided into 25 10 cm sub-squares was then placed with the sample plant at its centre. The size of the quadrat (50 cm) was chosen based on a study on a different Welsh E. aurinia population in which more than 90% of pre-diapause larval groups dispersed over less than 25 cm; it was concluded from this study that a 50 cm quadrat is suitable to reflect the range of pre-diapause larvae (Early 2006). As a measurement that combines both abundance and distribution of S. pratensis around the central plant, the number of sub-squares containing parts of S. pratensis plants was recorded (i.e. max. 25; in the following ‘S. pratensis score’). Distance of the sample plant to the nearest shelter, usually a Molinia tussock, was also recorded to account for proximity to shelter as a potential oviposition cue. The size of the oviposition leaf was determined by measuring the total length and maximum width of the leaf; for the control plants, this measurement was taken from a leaf that matched the corresponding oviposition leaf in relative size (i.e., if the leaf with the egg batch(es) was amongst the smallest leaves on the plant, the control measurement was taken on one of the smallest leaves of the control plant etc.). In order to achieve one standard measurement of leaf size, length was multiplied by width; subsequently this product was log transformed to reduce variance (in the following ‘leaf size score’).
On completion of the oviposition data gathering, the canes marking the oviposition and control plants were replaced with bright yellow survey marks and underlying metal washers which were attached to the ground immediately north of each sample plant with 12 cm long iron nails. Survey marks were numbered for identification. This was done because the reserve is cattle grazed in summer and any canes left in place would likely have been destroyed or moved.
Microhabitat for pre-diapause larvae
In early September 2013, the sampling points were re-found with the help of GPS and a metal detector and the microhabitat around the larval webs resulting from the original oviposition sampling points was assessed. The parameters recorded were the same as in the oviposition investigation, excepting leaf size. Instead, the distance between original host plant and current position of the larval web (i.e. pre-diapause larval dispersal distance) was recorded. This was measured in a straight line regardless of the shape of the path of abandoned webbing leading to the current web. The original null model was retained; only the sward height was updated.
Rotational grazing exclosure
Our study coincided with the end of the first period of cattle exclusion from a parcel of land on the otherwise grazed study site (see above). This allowed us to split the oviposition sample plants into three groups to investigate the effects of this trial; those derived from the grazed bulk of the site, those from the grazing exclosure and those derived from a transect running along the edge of the newly created exclosure in June 2013 (‘grazed’, ‘fenced’ and ‘edge’ treatment, respectively).
Data analysis
Student’s t test was used to compare sward heights, leaf size scores and distance to nearest shelter between oviposition or larval web and control plants. Where the assumption of equal variances was violated, a t test with a Satterthwaite approximation for the degrees of freedom replaced the Student’s test. S. pratensis scores around occupied and control plants were compared using a Mann–Whitney U test. Differences between ‘grazed’, ‘fenced’ and ‘edge’ sampling points of the oviposition investigation were tested with a combination of one-way analysis of variance (ANOVA) and Tukey’s test. All calculations were performed using SPSS Statistics 20.
Results
Microhabitat for oviposition
A total of 150 oviposition plants (= 150 sampling points) were located, some of which hosted two, three or four egg batches. These were compared with 150 control plants. At the control sampling points, the average vegetation height was 10.5 cm whilst the average oviposition plant was surrounded by a lower sward of 9.2 cm. The difference is statistically highly significant [t(298) = − 3.85, p < 0.001, see Fig. 1).
Around oviposition plants, the average S. pratensis score was 18.4 whilst the average around control plants was 12.3. The distribution of S. pratensis scores is shown in Fig. 2. Although the control data suggest that plants with fewer than nine surrounding sub-squares containing parts of S. pratensis are fairly frequent (28.7%), no egg batches were found on such plants. At the upper end of the distribution, none of the control plants scores higher than 23 whilst more than 5% of the egg batches were found on plants with a score of 24 or 25. The difference between the two sets of data is statistically highly significant (U = 3,975, p < 0.001), indicating an oviposition selection preference for S. pratensis plants with a greater density of surrounding S. pratensis plants than would be expected by random selection.
Based on leaf size scores, there was no difference in size between oviposition and control leaves [oviposition: 1.23, control: 1.28; t(298) = − 1.83, p = 0.068]. There was also no difference between oviposition and control plants in distance to the nearest Molinia tussock or other vegetation feature likely to provide suitable shelter for hibernation [oviposition: 19.0 cm, control: 17.4 cm; t(298) = 1.05, p = 0.293].
Microhabitat for pre-diapause larvae
On return to the study area in September, after the survey markers had been exposed to wind, weather, and cattle, it was not possible to revisit all 300 sampling points. In addition, some egg batches did not appear to have resulted in larval web establishment. The total sample size of the larval web investigation was 134 larval webs and 131 control plants. As not all of the assessments were updated for the control in September, the control sample size is 134 for the S. pratensis score and the distance to the nearest tussock (only the control points corresponding with the investigated larval webs (n = 134) were included in statistical analyses).
Sixteen oviposition plants could not be related to any resulting larval webs. In eight cases, this was because the survey marker could not be physically located. On the other eight occasions, the survey marker was found but the egg batches or webs were absent, either for no apparent reason or because the host plant had obviously been eaten by cattle. Out of the remaining sampling points, there were nine abandoned webs without larvae (these were still included in the larval microhabitat investigation). Assuming that the larvae had died in these cases, and assuming that larvae originating from different egg batches on the same host plant had merged into one group (Liu et al. 2006), mortality recorded on a communal level from the egg stage to the larval stage in early September was 12% (17 out of 142).
The larvae were found to have moved to locations surrounded by higher vegetation, both in comparison to the oviposition plants and in comparison to the surrounding sward in September. On average, larval webs were surrounded by 11.7 cm tall vegetation whilst the sward at the control plants was 9.2 cm. This difference is statistically highly significant [t(221) = 5.78, p < 0.001; see Fig. 3]. The average control sward height had decreased from July to September (from 10.5 to 9.2 cm) due to grazing. A general increase in vegetation height is therefore not the reason for the change in sward height from oviposition to larval stage.
Whilst S. pratensis presence on a small scale around the host plant appeared to be a requirement for oviposition, this seemed not to be the case for larval web establishment. Particularly remarkable is the high proportion of S. pratensis scores under five around larval webs in comparison to the control data (see Fig. 4). Larval groups do not tend to defoliate entire S. pratensis plants on the study site but rather leave intact the leaves’ midribs so that the plant is still recognisable; larval defoliation can hence be excluded as the reason for this difference. On average, the surrounding score was 10.8 for larval webs and 12.0 for the control. The difference is significant (U = 7,453, p = 0.016).
Larval groups had on average travelled 62 cm by early September. The maximum dispersal distance recorded was 2.4 m. On some occasions there was no conclusive path connecting oviposition plants to larval webs. In these cases, preference was given to the larval web nearest to the natal plant.
Rotational grazing exclosure
Out of the combined oviposition and control sampling points (n = 300), 234 were located in the grazed area, 50 in the fenced area and 16 along the edge of a newly created exclosure. The preference for oviposition plants with high surrounding S. pratensis scores was constant across all three treatments. However, the sward was significantly taller in the fenced area and the S. pratensis leaves found in this area were significantly larger (based on leaf size score) than those in the other two treatment areas (see Table 1). The transect with the highest oviposition plant density of all transects was located in the fenced area, although no significant difference could be shown in comparison to the counts derived from grazed transects due to a very small sample size for fenced transects (n = 2).
Discussion
Our results show that the microhabitats utilised for oviposition on the one hand and pre-diapause larval web establishment on the other hand differ significantly in terms of sward height and host plant cover. This indicates that the common practise of regarding pre-diapause larval web location as a proxy for oviposition location may need refinement. The larval groups in our study dispersed over greater distances than reported anywhere in the literature and this was the case for grazed, fenced and edge treatment areas. Whilst final instar post-diapause larvae are known to be very mobile (Stefanescu et al. 2006; Porter and Ellis 2011), records of long dispersal distances appear very unusual in pre-diapause larvae; however, there is anecdotal evidence that fairly long distance dispersals are not unusual for the study population (D Wheeler & D Woolley pers comm) and they also appear to take place in at least some other Welsh populations (JP pers obs).
The reasons for this are likely to be complex. It has been reported that E. aurinia females show a preference for relatively large host plants or relatively large leaves in other parts of the species’ range (Anthes et al. 2003; Liu et al. 2006). This strategy offers the advantage of deferring the need for pre-diapause larvae to move on to neighbouring host plants and hence the threat of dying in the risky process of dispersal (Gibbs and van Dyck 2009). It does indeed appear common in some populations for the larval group to remain on the natal host plant well into late summer. In a study of E. aurinia in Germany, 97.3% of larval groups had stayed on their original host plant by the end of August (Anthes et al. 2003). In a Chinese study, Liu et al. (2006) found that more than 60% of the larval groups were still on the original Scabiosa tschiliensis plant by September (S. tschiliensis is similar to S. pratensis). In contrast, only 3 of the 134 larval webs (2.2%) in this study were still located on the natal host plant by September. The direct comparison of oviposition plants to control plants in their immediate vicinity did not reveal a preference for larger host plants; however, the fact that plants selected for oviposition were at their highest density along one of the transects in the fenced off grazing exclosure where the host plant leaves were larger than on the remainder of the site may indicate a preference for larger host plants in areas where such a preference can be expressed (i.e., where larger plants exist).
The size structure in the host plant population appeared fairly homogenous across most of the study site which may be a result of a long period of extensive grazing pressure. Liu et al. (2006) found that larger, preferred oviposition plants were located in areas where only light mule grazing, but no cattle or sheep grazing was allowed during the summer and it appeared that the host plants grew larger as a consequence. Regardless of host plant size, our results clearly show an oviposition preference for host plants with high surrounding S. pratensis cover which supports the findings of other studies (e.g. Konvička et al. 2003; Betzholtz et al. 2007; Smee et al. 2011). In the absence of large host plants on much of the site, our study population appears to have developed a strategy of utilising many subsequent small host plants and dispersing over great distances as a consequence. E. aurinia larvae would probably not be able to disperse over great distances everywhere across the species’ range. Liu et al. (2006) reported that larvae in China died very quickly if grounded due to exposure to the hot soil. The weather in Wales in contrast is usually mild enough for larvae to travel short distances on the ground.
The preference shown by ovipositing females for host plants surrounded by relatively low vegetation (9.2 cm) is in line with a range of previous studies on E. aurinia habitat requirements for oviposition (Liu et al. 2006; Anthes et al. 2003; Konvička et al. 2003). A low surrounding sward is likely to increase the eggs’ and larvae’s exposure to the sun and thus offer a favourable microclimate for larval growth (Porter 1981). An oviposition preference for plants exposed to the sun has been shown by Anthes et al. (2003). E. aurinia larvae are known for their basking behaviour in sunny weather and it has been suggested that the opportunity to bask reduces the infection rate of post-diapause larvae with parasitoid braconid wasps such as Cotesia spp. (Porter 1983). In addition, short swards have been found to be correlated with higher S. pratensis densities than taller swards (Preston et al. 2008) so that the significant preference for lower swards may partly be an indirect effect of a preference for areas of high S. pratensis density. It has also been suggested that increased physical accessibility of the host plant is a factor in E. aurinia’s oviposition preference for lower swards (Anthes et al. 2003). Renwick and Chew (1994) pointed out that sward height also affects host plant prominence. Low surrounding vegetation enables the female to visually track potential hosts. It is important to stress that our findings do not suggest an ideal oviposition sward height in absolute terms, but rather a preference for host plant individuals in a sward that is relatively low in comparison to the immediate vicinity. Whilst the overall mean sward height at oviposition plants was 9.2 cm, occupied host plants in the ‘fenced’ area were in an average sward of 12.6 cm and oviposition plant density was highest in this area. This in combination with the marked difference in sward height between oviposition plants and pre-diapause larval webs underlines the vital importance of high structural variability on a small scale to optimise habitat carrying capacity and hence long-term conservation success (see Thomas et al. 2011).
Swards surrounding larval webs in early September in contrast were significantly taller than those surrounding control plants, suggesting that larvae, since hatching in July, had actively moved to areas of higher vegetation as they approach the time for entering hibernation. The larval preference for relatively high vegetation is in line with Smee et al. (2011) who found a preference for relatively higher swards in autumn and also with Botham et al. (2011). Other previous studies suggest that E. aurinia sward height requirements are the same for oviposition and for larval web establishment (Anthes et al. 2003; Konvička et al. 2003). This is not surprising, taking into account that larval dispersal distances recorded in these studies were low. A possible reason for a larval preference for a higher sward is shelter. E. aurinia larvae are known to create a web for hibernation in sheltered positions like the base of a tussock, where they are safe from grazing and extreme temperatures (Porter 1981). Borsje (2005) mentions anecdotal evidence of an oviposition preference for S. pratensis plants on the periphery of a tussock or tussocky area, arguing that such plants are protected against heavy grazing whilst still getting enough light to grow to large size. In our study, there was no significant difference between oviposition and control plants’ distance to the nearest tussocky structure. However, this does not necessarily indicate the absence of such a relationship. The study area has a varied sward structure so that the nearest tussock to any random plant is usually not far away and it would hence be difficult to prove a preference for plants with nearby tussocks, even if it does exist.
For Wales, a standardised habitat condition assessment has been developed in which habitats are assigned to habitat quality categories and mapped on a patch scale (Fowles 2005). The assessment is mainly based on overall sward height and abundance of S. pratensis as indicators of habitat quality. In order to qualify for the best quality category ‘Good Condition’, average sward height of a patch has to be in the range of 12–25 cm. The findings of this study indicate that it may be appropriate to decrease the lower limit of this range to account for oviposition requirements, subject to verification of the results at other sites. Host plant cover in the immediate vicinity of the oviposition site was a significant factor in oviposition selection in our study. E. aurinia clearly avoids host plant individuals in a secluded position, giving preference to plants within patches of S. pratensis. Larval webs in contrast were found in locations with much lower surrounding S. pratensis cover. In comparing S. pratensis requirements of ovipositing females and larval groups, this study clearly indicates that the importance of surrounding S. pratensis availability decreases from the egg to the late pre-diapause larval stage. Although larval groups in Wales typically do not enter hibernation before around late September, some occupied larval webs in this study were already found in tussocky vegetation, independent of S. pratensis, in early September. Further study could reveal how important surrounding S. pratensis cover and other micro-habitat characteristics are for post-diapause larvae which were not investigated in this study. Further study would also be required to determine at what stage larval groups in populations with long distance pre-diapause dispersals leave their natal host plant, i.e. up to which point in time larval group location can be confidently used as a proxy for oviposition location. Most importantly, we would recommend further research into the merits of rotational grazing exclosure; while it is well-established that grazing is vitally important to maintain overall habitat suitability, our findings may indicate that a much more dynamic grazing regime with varying stocking densities on a spatial and temporal scale may maximise habitat variability on a small scale and hence habitat carrying capacity and resilience. Our results clearly underline the importance of studying the autecology of E. aurinia on regional scales and for all the different habitats and host plants utilised by the species to inform evidence-based, locally adapted conservation management as a means of safeguarding this threatened species.
References
Anthes N, Fartmann T, Hermann G, Kaule G (2003) Combining larval habitat quality and metapopulation structure—the key for successful management of pre-alpine Euphydryas aurinia colonies. J Insect Conserv 7:175–185
Betzholtz P-E, Ehrig A, Lindeborg M, Dinnétz P (2007) Food plant density, patch isolation and vegetation height determine occurrence in a Swedish metapopulation of the marsh fritillary Euphydryas aurinia (Rottemburg, 1775) (Lepidoptera, Nymphalidae). J Insect Conserv 11:343–350
Borsje HJ (2005) The marsh fritillary butterfly in the Avalon Marshes, Somerset: a study on habitat restoration and the re-establishment potential (English Nature Research Reports, 632). English Nature, Peterborough
Botham MS, Ash D, Aspey N, Bourn NAD, Bulman CR, Roy DB, Swain J, Zannese A, Pywell RF (2011) The effects of habitat fragmentation on niche requirements of the marsh fritillary, Euphydryas aurinia (Rottemburg, 1775) on calcareous grasslands in southern UK. J Insect Conserv 15:269–277
Bulman CR (2001) Conservation biology of the marsh fritillary butterfly Euphydryas aurinia. Dissertation, University of Leeds
Bulman CR, Wilson RJ, Holt AR, Gálvez Bravo L, Early RI, Warren MS, Thomas CD (2007) Minimum viable metapopulation size, extinction debt, and the conservation of a declining species. Ecol Appl 17:1460–1473
BUTT/Butterflies Under Threat Team (1986) The management of chalk grassland for butterflies (Focus on Nature Conservation, 17). JNCC, Peterborough
Butterfly Conservation (2004) The marsh fritillary needs your help; a guide to managing damp grassland. Butterfly Conservation, Wareham
CCW (2008) Core management plan for Rhos Llawr Cwrt SAC. Countryside Council for Wales, Bangor
Dennis RLH, Hodgson JG, Grenyer R, Shreeve TG, Roy DB (2004) Host plants and butterfly biology. Do host-plant strategies drive butterfly status? Ecol Entomol 29:12–26
Early R (2006) Conservation at the landscape scale: the marsh fritillary and other British butterflies. Dissertation, University of York
Eichel S, Fartmann T (2008) Management of calcareous grasslands for Nickerl’s fritillary (Melitaea aurelia) has to consider habitat requirements of the immature stages, isolation, and patch area. J Insect Conserv 12:677–688
Ellis S, Bourn NAD, Bulman CR (2012) Landscape-scale conservation for butterflies and moths: lessons from the UK. Butterfly Conservation, Wareham
Fowles AP (2005) Habitat quality mapping for marsh fritillary populations (Staff science report 05/5/1). Countryside Council for Wales, Bangor
Fowles AP (2011) Metapopulation studies of the marsh fritillary Euphydryas aurinia: the need for interconnected grassland landscapes
Fowles AP, Smith R (2006) Mapping the habitat quality of patch networks for the marsh fritillary Euphydryas aurinia (Rottemburg, 1775) (Lepidoptera, Nymphalidae) in Wales. J Insect Conserv 10:161–177
Fox R, Asher J, Brereton T, Roy D, Warren M (2006) The state of butterflies in Britain and Ireland. Pisces Publications, Berkshire
Fox R, Brereton TM, Asher J, August TA, Botham MS, Bourn NAD, Cruickshanks KL, Bulman CR, Ellis S, Harrower CA, Middlebrook I, Noble DG, Powney GD, Randle Z, Warren MS, Roy DB (2015) The state of the UK’s butterflies 2015. Butterfly Conservation and the Centre for Ecology & Hydrology, Wareham
García-Barros E, Fartmann T (2009) Butterfly oviposition: sites, behaviour and modes. In: Settele J, Shreeve TG, Konvička M, van Dyck H (eds) Ecology of butterflies in Europe. Cambridge University Press, Cambridge, pp 29–42
Gibbs M, van Dyck H (2009) Reproductive plasticity, oviposition site selection, and maternal effects in fragmented landscapes. Behav Ecol Sociobiol 64:1–11
Hobson R, Bourn N, Warren M (2002) Conserving the marsh fritillary in Britain. Br Wildl 13:404–411
Konvička MV, Hula V, Fric Z (2003) Habitat of pre-hibernating larvae of the endangered butterfly Euphydryas aurinia (Lepidoptera: Nymphalidae): what can be learned from vegetation composition and architecture? Eur J Entomol 100:313–322
Liu W, Wang Y, Xu R (2006) Habitat utilization by ovipositing females and larvae of the marsh fritillary (Euphydryas aurinia) in a mosaic of meadows and croplands. J Insect Conserv 10:351–360
Porter K (1981) The population dynamics of small colonies of the butterfly Euphydryas aurinia. Dissertation, Oxford University
Porter K (1982) Basking behaviour in larvae of the butterfly Euphydryas aurinia. Oikos 38:308–312
Porter K (1983) Multivoltinism in Apanteles bignelli and the influence of weather on synchronisation with its host Euphydryas aurinia. Entomol Exp Appl 34:155–162
Porter K (1992) Eggs and egg-laying. In: Dennis RLH (ed) The ecology of butterflies in Britain. Oxford University Press, Oxford, pp 46–72
Porter K, Ellis S (2011) Securing viable metapopulations of the marsh fritillary butterfly, Euphydryas aurinia, (Lepidoptera: Nymphalidae) in Northern England. J Insect Conserv 15:111–119
Preston JS, McEvoy P, Reid N (2008) Monitoring of Devil’s-bit scabious as a likely means of assessing the conservation status of the marsh fritillary butterfly (Quercus project QU03-17). Environment & Heritage Service, Northern Ireland
Ravenscroft NOM, Gaywood MJ (1996) The status and habitat of the marsh fritillary butterfly in western Scotland (Research, Survey and Monitoring Report 21). Scottish Natural Heritage, Edinburgh
Renwick JAA, Chew FS (1994) Oviposition behavior in Lepidoptera. Annu Rev Entomol 39:377–400
Resetarits WJ (1996) Oviposition site choice and life history evolution. Am Zool 36:205–215
Saarinen K, Jantunen J, Valtonen A (2005) Resumed forest grazing restored a population of Euphydryas aurinia (Lepidoptera: Nymphalidae) in SE Finland. Eur J Entomol 102(4):683–690
Schtickzelle N, Choutt J, Goffart P, Fichefet V, Baguette M (2005) Metapopulation dynamics and conservation of the marsh fritillary butterfly: population viability analysis and management options for a critically endangered species in Western Europe. Biol Conserv 126:569–581
Smee M, Smyth W, Tunmore M, Ffrench-Constant R, Hodgson D (2011) Butterflies on the brink: habitat requirements for declining populations of the marsh fritillary (Euphydryas aurinia) in SW England. J Insect Conserv 15:153–163
Stefanescu C, Peňuelas J, Sardans J, Filella I (2006) Females of the specialist butterfly Euphydryas aurinia (Lepidoptera: Nymphalinae: Melitaeini) select the greenest leaves of Lonicera implexa (Caprifoliaceae) for oviposition. Eur J Entomol 103:569–574
Stewart KEJ, Bourn NAD, Thomas JA (2001) An evaluation of three quick methods commonly used to assess sward height in ecology. J Appl Ecol 38:1148–1154
Thomas JA, Simcox DJ, Hovestadt T (2011) Evidence based conservation of butterflies. J Insect Conserv 15:241–258
Tjørnløv RS, Kissling WD, Barnagaud J-Y, Bøcher PK, Høye T (2015) Oviposition site selection of an endangered butterfly at local spatial scales. J Insect Conserv 19:377–391
van Swaay CAM, Warren MS (1999) Red data book of European butterflies (Rhopalocera) (Nature and Environment 99). Council of Europe Publishing, Strasbourg
Warren MS (1994) The UK status and suspected metapopulation structure of a threatened European species, Eurodryas aurinia. Biol Conserv 67:239–249
Acknowledgements
Many thanks to Adrian Fowles and the late David Wheeler for their technical support and critical feedback, as well as to the volunteers in the field Elsa Davies, Ceri Owen-Jones and Geoff Liles. Caroline Bulman and Russel Hobson provided helpful comments on the text. JP acknowledges a scholarship from the German Academic Scholarship Foundation. The write-up was supported by Butterfly Conservation. Natural Resources Wales gave permission to carry out fieldwork on Rhos Llawrcwrt (the study was non-invasive and no specimens were taken).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Pschera, J., Warren, J.M. Microhabitat selection by ovipositing females and pre-diapause larvae of a Welsh population of Euphydryas aurinia (Lepidoptera: Nymphalidae). J Insect Conserv 22, 571–579 (2018). https://doi.org/10.1007/s10841-018-0086-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-018-0086-4