Abstract
Background
Vascular complications are a common occurrence during atrial fibrillation ablation. Observational studies indicate that the utilization of ultrasound (US)-guided puncture may decrease the incidence of vascular complications; however, its routine use is not established in many centres.
Methods
Patients undergoing catheter ablation for atrial fibrillation were included sequentially. All patients receiving US-guided punctures were prospectively enrolled (US group), while patients who underwent the procedure with standard puncture technique served as control group (No-US group). Periprocedural vascular complications requiring intervention within 30 days of the procedure were defined as the primary endpoint.
Results
A total of 599 patients (average age: 69 ± 11 years, 62.9% male) were analysed. The incidence of vascular complications was lower with the US-guided puncture than with the anatomic landmark-guided puncture (14/299 [4.7%] vs. 27/300 [9%], p = 0.036). The US-guided vascular access significantly reduced the rate of false aneurysms (3/299 [1%] vs. 12/300 [4%], p = 0.019). In addition, the occurrence of arteriovenous fistula (2/299 [0.7%] vs. 4/300 [1.3%], p = 0.686) and haematoma requiring treatment (9/299 [3%] vs. 11/300 [3.7%], p = 0.655) were also lower in the US group. US-guided puncture did not prolong the procedure time (mean procedure time: 57.48 ± 24.47 min vs. 56.09 ± 23.36 min, p = 0.478). Multivariate regression analysis identified female gender (OR 2.079, CI 95% 1.096–3.945, p = 0.025) and conventional vascular access (OR 2.079, CI 95% 1.025–3.908, p = 0.042) as predictors of vascular complications.
Conclusions
The implementation of US-guided vascular access for left atrial catheter ablation resulted in a significant decrease of the overall vascular complication rate.
Similar content being viewed by others
1 Introduction
Atrial fibrillation (AF) is the most common cardiac rhythm disorder affecting 2 to 4% of adults [1]. In recent decades, there has been a significant increase in the number of AF ablation procedures [2]. Catheter ablation is a well-established treatment option to reduce arrhythmia-related symptoms and improve quality of life [1]. The most frequent complications of AF ablation are related to vascular access, with a reported incidence varying from 2 to 4% according to the latest European Society of Cardiology guideline [1]. Continuous anticoagulation, intraprocedural systemic anticoagulation and the use of multiple large-diameter sheaths during AF ablation increase the risk of bleeding [3]. Conventionally, femoral venous access was achieved by ‘blind’ puncture medial to where the femoral arterial pulse was palpated. However, computer tomography scans showed overlapping femoral artery and vein in two-thirds of patients [4]. To facilitate vascular access, real-time ultrasound (US) guidance is recommended in many medical specialities, including anaesthesiology [5], intensive care [6], nephrology [7] and paediatrics [8]. However, routine use of US-guided vascular access is not established in many electrophysiology centres. Furthermore, a prospective randomised study found no clear advantage of US-guided venous puncture in reducing vascular access complications [9]. The present study investigated the impact of US-guided venous access for left atrial (LA) ablation procedures in a high-volume ablation centre.
2 Materials and methods
The study was approved by the local ethics committee (2022–2956-evBO) and complies with the Declaration of Helsinki.
Patients undergoing catheter ablation for LA arrhythmias were enrolled consecutively at a single high-volume ablation centre (1400 AF catheter ablations per year). All patients undergoing US-guided vascular access between August and October 2022 were prospectively enrolled (US group). Patients who underwent the procedure with anatomic landmark-guided puncture between September and November 2021 served as control group (No-US group). Before the investigation had started, anatomic landmark-guided cannulation was the standard puncture technique in our institution. The US-guided venous access was introduced for this study.
2.1 Study participants
All patients over 18 years of age with AF or LA tachycardia were eligible to enter the study. Participants in the US group had to sign the patient informed consent form before enrolment. Planned arterial puncture for the procedure led to exclusion from the study.
2.2 Anticoagulation
Novel oral anticoagulation (NOAC) therapy was minimally interrupted by skipping the morning dose on the day of the procedure and resuming NOAC therapy six hours after the procedure. Uninterrupted vitamin K antagonist therapy with an INR value between 2 and 3 was aimed. In patients naive to oral anticoagulation (OAC), NOAC therapy was started six hours after ablation.
2.3 Ablation procedure
All ablation procedures were performed under deep intravenous sedation using midazolam, fentanyl and propofol. The right femoral vein was used to gain venous access. After palpation of the arterial pulse and application of local anaesthesia, the physician obtained vascular access with the Seldinger technique. Depending on the ablation modality, inserted sheaths differed in size and number. Standard radiofrequency (RF) ablation required three sheaths with an inner diameter (ID) of 8,5 French (Fr). Standard single-shot ablation required one 8 Fr and one large-diameter sheath (12–13.8 Fr ID). After venous access, intravenous unfractionated heparin was administered according to the body weight to maintain an activated clotting time of at least 300 s. No protamine was administered at the end of the ablation. The catheters and sheaths were removed and the femoral vein was routinely sealed with a Z-suture. Pressure tape was rarely applied according to the operator's preference, particularly in cases of immediate post-procedural bleeding, inadvertent arterial puncture, in extremely thin patients or additionally in the case of insufficient Z-suture.
This represents the standard workflow at our institution. The procedure and peri-procedural patient management were identical in both groups.
2.4 US-guided puncture
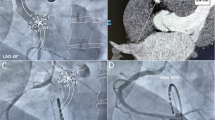
A linear array probe was covered with a sterile sheath for US-guided puncture. The probe was positioned below the inguinal ligament at a 90-degree angle to the femoral vein, displaying the vessels in short axis (Fig. 1a). The venous image was placed in the middle of the screen. The femoral vein is a compressible structure typically located medial to the non-compressible femoral artery. To avoid an inadvertent arterial puncture, the artery and vein should be side by side, not on top of each other (Fig. 1b, c). The needle tip or at least the tissue moving should be visible when penetrating the venous wall.
US-guided venous access. a Two-handed puncture technique. b The optimal puncture site with the femoral vein situated medial to the femoral artery and visualisation of the needle just before entering the vein (white arrow). c The suboptimal puncture site with the femoral vein located inferior to the femoral artery. FA, femoral artery; FV, femoral vein
Before starting the study, all physicians performed at least ten roll-in cases with US-guided vascular access. Training cases were excluded from the analysis. All physicians were experienced in the use of anatomic landmark-guide puncture.
2.5 Endpoints
The primary endpoints were vascular complications, including pseudoaneurysm, arteriovenous (AV) fistula or haematoma requiring treatment such as transfusion, surgery, prolonged mechanical compression, analgesics or extended hospitalisation, within 30 days. Prolonged compression is defined as secondary haemorrhage on the ward requiring continued compression after primary Z-suture or pressure tape. Major vascular complications encompassed complications that resulted in extended hospitalisation, readmission or required intervention such as surgery or transfusion. When participants were diagnosed with multiple complications, only the most relevant complication was analysed (vascular complications relevance: pseudoaneurysm > AV fistula > haematoma).
Secondary endpoints were the procedure time and days of hospitalisation.
2.6 Post-procedural management and follow-up
OAC was resumed six hours after ablation. Before discharge, patients underwent daily clinical examinations, including inspection, palpation and auscultation of the puncture side. A colour duplex sonography was performed in the event of abnormalities in the clinical examination. All examination results were documented in the electronic patient record. Post-procedural inpatient monitoring was planned for two nights. After discharge, all patients were instructed to return to our outpatient clinic in case of any vascular complications. For a period of 30 days after hospital discharge, each clinical visit for a vascular complication was followed up.
2.7 Statistic
Continuous variables were presented as mean and standard deviation or median and interquartile range. A two-sided t test for independent samples was used to compare continuous variables with normal distribution. In case of variance heterogeneity, the Mann–Whitney U test was performed. Categorical variables were summarised as frequencies and percentages and compared with the Chi-square or Fischer’s exact test.
Binary logistic regression, expressed as odds ratio (OR) with 95% confidence interval (CI), was performed to identify independent predictors of vascular complications. Considering the interaction of various risk factors, a multivariable model was constructed including the following covariates: Body mass index (BMI), female gender, RF ablation and conventional vascular access. A p-value < 0.05 was considered to indicate statistical significance. Statistical analysis was performed by using the SPSS statistical package, version 29.0.
3 Results
3.1 Patients
Overall, 600 patients were included, 300 per group. One patient in the US group was excluded due to an intended arterial puncture on the venous access site, leaving 299 patients for analysis. In the US group, one patient developed a haematoma after the initial puncture, which made it impossible to identify the vessels on the US image. Therefore, the last two punctures were performed using anatomic landmark-guided puncture.
3.2 Baseline characteristics
Demographic and clinical baseline characteristics were well-matched between the two groups, except for a higher prevalence of pre-existing diabetes mellitus in the control group (14.7% vs. 8.7%, p = 0.023) and higher INR values (1.2 [1.09–1.36]) vs. 1.23 [1.1–1.5], p = 0.043) in the US group. Detailed patient characteristics are summarised in Table 1. Most patients were male (62.9%), on average 69 years old, and the vast majority of patients were receiving NOACs (85%).
In 51.4% and 48.6% of patients, point-by-point RF and single-shot ablation were performed, respectively (p = 0.513). For single-shot modalities, pulsed field ablation (41.9%), cryoballoon (39.9%), RF balloon (13.7%) and laserballoon (4.5%) were used. Vascular access was most frequently closed with a Z-suture (No-US: 93% vs. US: 96.3%, p = 0.071). In a minority of cases, pressure tape was used to seal the access site (5.3% vs. 3.7%, p = 0.329), including six patients in whom Z-suture was initially used for vascular occlusion. In the No-US group, a vascular closure device was used in five patients to close vascular access.
3.3 Study endpoints
The primary outcome measure—the rate of vascular complications within 30 days of the ablation procedure—is shown in Table 2. US-guided access was associated with a significant reduction in the overall incidence of vascular complications (4.7% vs. 9%, p = 0.036) and the rate of false aneurysms (1% vs. 4%, p = 0.019). In addition, the incidence of AV fistula (0.7% vs. 1.3%, p = 0.686) and haematoma requiring treatment (3% vs. 3.7%, p = 0.655) was numerically lower in the US group.
US-guided vascular access must be performed in 24 patients to prevent one vascular complication. The rate of major vascular complications was significantly reduced with US-guided venous access (1% vs. 6.3%, p = < 0.001).
The way the different types of complications were managed is illustrated in Fig. 2. Interventional management was required in all 15 patients diagnosed with pseudoaneurysm. The six patients with AV fistulas were treated with pressure tape. In the case of a haematoma, interventions included prolonged compression, prolonged hospital stay and analgesics for groin pain. Pressure tape was consistently applied for prolonged compression, except in one case using a femoral compression system called ‘FemoStop’. Transfusions and surgery were required only in two patients with a pseudoaneurysm, who were finally treated with coil embolisation.
Secondary endpoint analysis showed that days of hospital stay were significantly lower in the US group (2.02 ± 0.61 vs. 2.18 ± 1.22, p = 0.043). Moreover, US guidance did not prolong the procedure time (57.48 ± 24.47 min vs. 56.09 ± 23.36 min, p = 0.478).
3.4 Predictors of vascular complication
As demonstrated in Table 3, univariate regression analysis identified female gender (OR 2.073, CI 95% 1.095–3.922, p = 0.025) and conventional vascular access (OR 2.013, CI 95% 1.034–3.921, p = 0.04) as predictors of vascular complications. On multivariate regression analysis, female gender (OR 2.079, CI 95% 1.096–3.945, p = 0.025) and conventional vascular access (OR 2.079, CI 95% 1.025–3.908, p = 0.042) were identified as independent predictors of vascular complications.
4 Discussion
Vascular access complications remain the most common complication of AF ablation procedures [10, 11]. Although most often not life-threatening, it may be associated with increased consumption of health care resources including interventional treatment, surgery, and prolonged hospitalisation.
A most recent study suggested that the use of US did not significantly reduce the number of procedure-related complications for experienced operators [9]. However, the study was halted prematurely due to an unexpectedly low complication rate.
The current study found that the implementation of US significantly reduced the incidence of complications by approximately 50%. Expectedly, female gender was identified as another independent predictor of access site complications, doubling the risk for a complication.
4.1 Incidence of primary endpoints
Heterogeneous definitions contribute to the wide variations of reported incidences of vascular complications, which range from 2 to 14% [12, 13]. This study considered a wider range of endpoints, including haematomas resolved with compression or analgesics and conservatively treated AV fistulas, which explains a relatively high overall complication rate of 6.8%. Additionally, all data were collected within a 30-day follow-up period, whereas numerous register studies solely analysed inpatient events when reporting complication rates. However, the lack of routine 30-day follow-up may have underestimated the rate of vascular complications.
4.2 Applicability of results
In recent studies, female gender was identified as a risk factor for major complications in catheter ablation [14, 15]. Dugo et al. speculated that the smaller diameter of the vessels and the closer proximity of the femoral vessels in women may be a cause of the gender-related differences in vascular complications [16]. However, the advantages of using ultrasound for all patients and ablation strategies can still be inferred beyond just women. The current study confirmed previous findings, including a meta-analysis, that BMI is not an independent predictor of vascular access complications [17, 18]. Nonetheless, conventional venous access guided by femoral pulse palpation may be particularly challenging in obese patients. US guidance provides a sound solution to decrease the incidences of unsuccessful attempts, inadvertent arterial puncture and vascular complications [19, 20].
The number and size of the sheaths used for AF catheter ablation did not seem to be a significant factor in the incidence of groin complications. This confirms recent randomised studies comparing irrigated RF current ablation with single-shot devices, which are usually associated with large bore sheath access. In the Fire and Ice Trial, there was a non-significant difference in groin complications between the RF (4.3%) and the cryoballoon (1.9%) groups (p = 0.09). Similarly, in the laser versus RF for persistent AF study, no significant differences were found [21, 22].
Data on the risks associated with the placement of large-diameter venous sheats are provided by interventional valve reconstructions using 24 Fr sheats. There were higher rates of major vascular complications in mitral (3.5%) and tricuspid (11%) valve reconstruction [23, 24]. However, comparing the risks associated with AF ablation procedures is challenging due to continuous anticoagulation, intraprocedural systemic anticoagulation, and the use of multiple sheats during AF ablation.
4.3 Complication management
Pseudoaneurysm was the most common complication after conventional venous puncture, leading to interventional treatment in all cases. These interventions (i.e. thrombin injection, coil embolisation and stent implantation) are associated with additional risks for complications [25]. In addition, complication management also contributes to higher healthcare expenditures resulting in a worse cost–benefit ratio of AF catheter ablation [26]. Bode and co-workers conducted an analysis that revealed a rise of costs from €139.54 to €153.31 per vascular complication, primarily attributable to the occurrence of pseudoaneurysm and longer hospital stay. In this study and further investigations, these parameters were reduced in the US group [27]. Therefore, implementing US-guided vascular access has a positive economic impact by reducing the incidence of vascular complications.
Uninterrupted OAC substantially reduces the risk for peri-procedural stroke [28]. Research has shown that the risk of stroke after ablation increases within the initial 30 days [29]. In the event of bleeding, the cessation of OAC often increases the risk of stroke. This highlights the importance of secure vascular access to enable uninterrupted stroke prophylaxis.
4.4 Impact on work-flow with focus on same-day discharge
Work-flow optimisation is key to treat a growing number of patients effectively. Implementation of US-guided venous access is associated with minimal added expense for a sterile cover and should be available at almost all hospitals. Additionally, it does not lead to prolonged procedure times and has been demonstrated to actually shorten puncture time in other studies [17, 30]. In the study referred to above, the use of US resulted in lower puncture time, as well as a reduction in the number of inadvertent arterial punctures and puncture attempts. These findings were consistent for both trainees and expert operators, indicating that the introduction of US-guided vascular access has a short learning curve [31].
Recently, several observational studies suggested that AF ablation procedures based on a same-day discharge strategy are feasible and safe [32]. The absence of vascular access complications plays a crucial role in this regard. It seems that in addition to a safe venipuncture, a vascular closure system can contribute to excellent clinical outcomes [33, 34]. This also translated into improved patient reported outcomes and reduced clinical costs [35].
4.5 Limitations
The main limitation was the non-randomised study design and the retrospectively collected data of the control group. To minimise the selection bias, all patients were enrolled consecutively. However, this study design resulted in a difference between the groups concerning the prevalence of diabetes mellitus and in the INR levels. Although there was a significant difference in INR values in the analysis, this slight dissimilarity is not clinically relevant and instead would increase the risk of bleeding in the US group. The effect of diabetes mellitus the rate of vascular complications was investigated in similar studies and diabetes mellitus was not an independent predictor of vascular complications [30, 36]. Therefore, it is unlikely that this is the reason for the differences in vascular complications. Moreover, there were group differences in relation to the operator who carried out the venous access.All venous punctures in the No-US group were performed by experienced operators, while three additional fellows were involved in the US group. Although more fellows performed vascular access in the US group, the rate of access-related complications could be reduced. In addition, all operators in this study were unfamiliar with US-guided venous puncture at the beginning of the analysis. This suggests that US-guided vascular access is easy to learn, even for inexperienced operators. Overall, there is a risk of confounding, as in all observational studies, which limits the validity of the conclusions. Independent physicians performed the follow-up and duplex US in case of complications to minimise observer bias. At least, conducting this investigation in a high-volume centre with a complex study population carries a risk of referral bias.
5 Conclusion
In a high-volume AF ablation centre, the introduction of US-guided venous access did result in a significant reduction of venous access site complications. An adequately powered randomised multi-centre trial is urgently needed.
Data availability
The raw data will be shared with other researchers on reasonable request.
References
Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, Fauchier L, Filippatos G, Kalman JM, La Meir M, Lane DA, Lebeau JP, Lettino M, Lip GYH, Pinto FJ, Thomas GN, Valgimigli M, Van Gelder IC, Van Putte BP, Watkins CL, ESC Scientific Document Group, Kirchhof P, Kühne M, Aboyans V, Ahlsson A, Balsam P, Bauersachs J, Benussi S, Brandes A, Braunschweig F, Camm AJ, Capodanno D, Casadei B, Conen D, Crijns HJGM, Delgado V, Dobrev D, Drexel H, Eckardt L, Fitzsimons D, Folliguet T, Gale CP, Gorenek B, Haeusler KG, Heidbuchel H, Iung B, Katus HA, Kotecha D, Landmesser U, Leclercq C, Lewis BS, Mascherbauer J, Merino JL, Merkely B, Mont L, Mueller C, Nagy KV, Oldgren J, Pavlović N, Pedretti RFE, Petersen SE, Piccini JP, Popescu BA, Pürerfellner H, Richter DJ, Roffi M, Rubboli A, Scherr D, Schnabel RB, Simpson IA, Shlyakhto E, Sinner MF, Steffel J, Sousa-Uva M, Suwalski P, Svetlosak M, Touyz RM, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, Fauchier L, Filippatos G, Kalman JM, La Meir M, Lane DA, Lebeau JP, Lettino M, Lip GYH, Pinto FJ, Neil Thomas G, Valgimigli M, Van Gelder IC, Watkins CL, Delassi T, Sisakian HS, Scherr D, Chasnoits A, Pauw MD, Smajić E, Shalganov T, Avraamides P, Kautzner J, Gerdes C, Alaziz AA, Kampus P, Raatikainen P, Boveda S, Papiashvili G, Eckardt L, Vassilikos V, Csanádi Z, Arnar DO, Galvin J, Barsheshet A, Caldarola P, Rakisheva A, Bytyçi I, Kerimkulova A, Kalejs O, Njeim M, Puodziukynas A, Groben L, Sammut MA, Grosu A, Boskovic A, Moustaghfir A, Groot N de, Poposka L, Anfinsen OG, Mitkowski PP, Cavaco DM, Siliste C, Mikhaylov EN, Bertelli L, Kojic D, Hatala R, Fras Z, Arribas F, Juhlin T, Sticherling C, Abid L, Atar I, Sychov O, Bates MGD, Zakirov NU. (2020) ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 42(5):373–498. https://doi.org/10.1093/eurheartj/ehaa612
Hindricks G, Shamloo AS, Lenarczyk R, Kalarus Z, Arya A, Kircher S, Darma A, Dagres N. Catheter ablation of atrial fibrillation: current status, techniques, outcomes, and challenges. Kardiologia Pol Pol Heart J. 2018;76(12):1680–6. https://doi.org/10.5603/KP.a2018.0216.
Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, Akar JG, Badhwar V, Brugada J, Camm J, Chen PS, Chen SA, Chung MK, Cosedis Nielsen J, Curtis AB, Davies DW, Day JD, d’Avila A, de Natasja Groot NMS, Di Biase L, Duytschaever M, Edgerton JR, Ellenbogen KA, Ellinor PT, Ernst S, Fenelon G, Gerstenfeld EP, Haines DE, Haissaguerre M, Helm RH, Hylek E, Jackman WM, Jalife J, Kalman JM, Kautzner J, Kottkamp H, Kuck KH, Kumagai K, Lee R, Lewalter T, Lindsay BD, Macle L, Mansour M, Marchlinski FE, Michaud GF, Nakagawa H, Natale A, Nattel S, Okumura K, Packer D, Pokushalov E, Reynolds MR, Sanders P, Scanavacca M, Schilling R, Tondo C, Tsao HM, Verma A, Wilber DJ, Yamane T. HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace. 2017;20(1):e1-160. https://doi.org/10.1093/europace/eux274.
Baum PA, Matsumoto AH, Teitelbaum GP, Zuurbier RA, Barth KH. Anatomic relationship between the common femoral artery and vein: CT evaluation and clinical significance. Radiology. 1989. https://doi.org/10.1148/radiology.173.3.2813785.
Rupp S. Practice Guidelines for Central Venous Access: A Report by the American Society of Anesthesiologists Task Force on Central Venous Access. Anesthesiology. 2012;116(3):539–73. https://doi.org/10.1097/ALN.0b013e31823c9569.
Lamperti M, Bodenham AR, Pittiruti M, Blaivas M, Augoustides JG, Elbarbary M, Pirotte T, Karakitsos D, LeDonne J, Doniger S, Scoppettuolo G, Feller-Kopman D, Schummer W, Biffi R, Desruennes E, Melniker LA, Verghese ST. International evidence-based recommendations on ultrasound-guided vascular access. Intensive Care Med. 2012;38(7):1105–17. https://doi.org/10.1007/s00134-012-2597-x.
Lok CE, Huber TS, Lee T, Shenoy S, Yevzlin AS, Abreo K, Allon M, Asif A, Astor BC, Glickman MH, Graham J, Moist LM, Rajan DK, Roberts C, Vachharajani TJ, Valentini RP. KDOQI Clinical Practice Guideline for Vascular Access: 2019 Update. Am J Kidney Dis. 2020;75(4):S1-164. https://doi.org/10.1053/j.ajkd.2019.12.001.
Aouad MT, Kanazi GE, Abdallah FW, Moukaddem FH, Turbay MJ, Obeid MY, Siddik-Sayyid SM. Femoral vein cannulation performed by residents: a comparison between ultrasound-guided and landmark technique in infants and children undergoing cardiac surgery. Anesth Analg. 2010;111(3):724. https://doi.org/10.1213/ANE.0b013e3181e9c475.
Yamagata K, Wichterle D, Roubíček T, Jarkovský P, Sato Y, Kogure T, Peichl P, Konečný P, Jansová H, Kučera P, Aldhoon B, Čihák R, Sugimura Y, Kautzner J. Ultrasound-guided versus conventional femoral venipuncture for catheter ablation of atrial fibrillation: a multicentre randomized efficacy and safety trial (ULTRA-FAST trial). EP Eur. 2018;20(7):1107–14. https://doi.org/10.1093/europace/eux175.
Packer DL, Mark DB, Robb RA, Monahan KH, Bahnson TD, Poole JE, Noseworthy PA, Rosenberg YD, Jeffries N, Mitchell LB, Flaker GC, Pokushalov E, Romanov A, Bunch TJ, Noelker G, Ardashev A, Revishvili A, Wilber DJ, Cappato R, Kuck KH, Hindricks G, Davies DW, Kowey PR, Naccarelli GV, Reiffel JA, Piccini JP, Silverstein AP, Al-Khalidi HR, Lee KL. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA Randomized Clinical Trial. JAMA - J Am Med Assoc. 2019;321(13):1261–74. https://doi.org/10.1001/jama.2019.0693.
Deshmukh A, Patel NJ, Pant S, Shah N, Chothani A, Mehta K, Grover P, Singh V, Vallurupalli S, Savani GT, Badheka A, Tuliani T, Dabhadkar K, Dibu G, Reddy YM, Sewani A, Kowalski M, Mitrani R, Paydak H, Viles-Gonzalez JF. In-hospital complications associated with catheter ablation of atrial fibrillation in the United States between 2000 and 2010: analysis of 93 801 procedures. Circulation. 2013;128(19):2104–12. https://doi.org/10.1161/CIRCULATIONAHA.113.003862.
Bohnen M, Stevenson WG, Tedrow UB, Michaud GF, John RM, Epstein LM, Albert CM, Koplan BA. Incidence and predictors of major complications from contemporary catheter ablation to treat cardiac arrhythmias. Heart Rhythm. 2011;8(11):1661–6. https://doi.org/10.1016/j.hrthm.2011.05.017.
Ghaye B, Szapiro D, Dacher JN, Rodriguez LM, Timmermans C, Devillers D, Dondelinger RF. Percutaneous ablation for atrial fibrillation: the role of cross-sectional imaging. RadioGraphics. 2003;23(suppl_1):S19-33. https://doi.org/10.1148/rg.23si035513.
Padala SK, Gunda S, Sharma PS, Kang L, Koneru JN, Ellenbogen KA. Risk model for predicting complications in patients undergoing atrial fibrillation ablation. Heart Rhythm. 2017;14(9):1336–43. https://doi.org/10.1016/j.hrthm.2017.04.042.
Spragg DD, Dalal D, Cheema A, Scherr D, Chilukuri K, Cheng A, Henrikson CA, Marine JE, Berger RD, Dong J, Calkins H. Complications of catheter ablation for atrial fibrillation: incidence and predictors. J Cardiovasc Electrophysiol. 2008;19(6):627–31. https://doi.org/10.1111/j.1540-8167.2008.01181.x.
Dugo D, Bordignon S, Perrotta L, Fürnkranz A, Julian Chun K, Schmidt B. Catheter ablation of atrial fibrillation in females. J Atr Fibrillation. 2013;6(1):893. https://doi.org/10.4022/jafib.893.
Wynn GJ, Haq I, Hung J, Bonnett LJ, Lewis G, Webber M, Waktare JEP, Modi S, Snowdon RL, Hall MCS, Todd DM, Gupta D. Improving safety in catheter ablation for atrial fibrillation: a prospective study of the use of ultrasound to guide vascular access: vascular ultrasound for AFA. J Cardiovasc Electrophysiol. 2014;25(7):680–5. https://doi.org/10.1111/jce.12404.
Triantafyllou K, Karkos CD, Fragakis N, Antoniadis AP, Meletidou M, Vassilikos V. Ultrasound-guided versus anatomic landmark-guided vascular access in cardiac electrophysiology procedures: a systematic review and meta-analysis. Indian Pacing Electrophysiol J. 2022;22(3):145–53. https://doi.org/10.1016/j.ipej.2022.01.005.
De Sensi F, Miracapillo G, Addonisio L, Breschi M, Scalese M, Cresti A, Paneni F, Limbruno U. Predictors of successful ultrasound guided femoral vein cannulation in electrophysiological procedures. J Atr Fibrillation. 2018;11(3):2083. https://doi.org/10.4022/jafib.2083.
Foerschner L, Erhard N, Dorfmeister S, Telishevska M, Kottmaier M, Bourier F, Lengauer S, Lennerz C, Bahlke F, Krafft H, Englert F, Popa M, Kolb C, Hessling G, Deisenhofer I, Reents T. Ultrasound-guided access reduces vascular complications in patients undergoing catheter ablation for cardiac arrhythmias. J Clin Med. 2022;11(22):6766. https://doi.org/10.3390/jcm11226766.
Kuck KH, Brugada J, Fürnkranz A, Metzner A, Ouyang F, Chun KRRJ, Elvan A, Arentz T, Bestehorn K, Pocock SJ, Albenque JP, Tondo C. FIRE AND ICE Investigators Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med. 2016;374(23):2235–45. https://doi.org/10.1056/NEJMoa1602014.
Schmidt B, Neuzil P, Luik A, OscaAsensi J, Schrickel JW, Deneke T, Bordignon S, Petru J, Merkel M, Sediva L, Klostermann A, Perrotta L, Cano O, Chun KRJ. Laser balloon or wide-area circumferential irrigated radiofrequency ablation for persistent atrial fibrillation: a multicenter prospective randomized study. Circ Arrhythm Electrophysiol. 2017;10:12. https://doi.org/10.1161/CIRCEP.117.005767.
Obadia JF, Messika-Zeitoun D, Leurent G, Iung B, Bonnet G, Piriou N, Lefèvre T, Piot C, Rouleau F, Carrié D, Nejjari M, Ohlmann P, Leclercq F, Saint Etienne C, Teiger E, Leroux L, Karam N, Michel N, Gilard M, Donal E, Trochu JN, Cormier B, Armoiry X, Boutitie F, Maucort-Boulch D, Barnel C, Samson G, Guerin P, Vahanian A, Mewton N. Percutaneous repair or medical treatment for secondary mitral regurgitation. N Engl J Med. 2018;379(24):2297–306. https://doi.org/10.1056/NEJMoa1805374.
Nickenig G, Weber M, Lurz P, von Bardeleben RS, Sitges M, Sorajja P, Hausleiter J, Denti P, Trochu JN, Näbauer M, Dahou A, Hahn RT. Transcatheter edge-to-edge repair for reduction of tricuspid regurgitation: 6-month outcomes of the TRILUMINATE single-arm study. The Lancet. 2019;394(10213):2002–11. https://doi.org/10.1016/S0140-6736(19)32600-5.
Peters S, Braun-Dullaeus R, Herold J. Pseudoaneurysm incidence, therapy and complications. Hamostaseologie. 2018;38:166–72.
Bode K, Ueberham L, Gawlik S, Hindricks G, Bollmann A. Inguinal vascular complications after ablation of atrial fibrillation: an economic impact assessment. EP Eur. 2019;21(1):91–8. https://doi.org/10.1093/europace/euy132.
La Greca C, Cirasa A, Di Modica D, Sorgato A, Simoncelli U, Pecora D. Advantages of the integration of ICE and 3D electroanatomical mapping and ultrasound-guided femoral venipuncture in catheter ablation of atrial fibrillation. J Interv Card Electrophysiol. 2021;61(3):559–66. https://doi.org/10.1007/s10840-020-00835-6.
Di BL, Burkhardt JD, Santangeli P, Mohanty P, Sanchez JE, Horton R, Gallinghouse GJ, Themistoclakis S, Rossillo A, Lakkireddy D, Reddy M, Hao S, Hongo R, Beheiry S, Zagrodzky J, Rong B, Mohanty S, Elayi CS, Forleo G, Pelargonio G, Narducci ML, Dello RA, Casella M, Fassini G, Tondo C, Schweikert RA, Natale A. Periprocedural stroke and bleeding complications in patients undergoing catheter ablation of atrial fibrillation with different anticoagulation nagement results from the role of coumadin in preventing thromboembolism in atrial fibrillation (AF) patients undergoing catheter ablation (COMPARE) randomized trial. Circulation. 2014;129(25):2638–44. https://doi.org/10.1161/CIRCULATIONAHA.113.006426.
Noseworthy PA, Kapa S, Deshmukh AJ, Madhavan M, Van Houten H, Haas LR, Mulpuru SK, McLeod CJ, Asirvatham SJ, Friedman PA, Shah ND, Packer DL. Risk of stroke after catheter ablation versus cardioversion for atrial fibrillation: a propensity-matched study of 24,244 patients. Heart Rhythm. 2015;12:1154–61. https://doi.org/10.1016/j.hrthm.2015.02.020.
Ströker E, de Asmundis C, Kupics K, Takarada K, Mugnai G, De Cocker J, Stockman D, Sieira J, Schwagten B, Brugada P, De Greef Y, Chierchia GB. Value of ultrasound for access guidance and detection of subclinical vascular complications in the setting of atrial fibrillation cryoballoon ablation. EP Eur. 2019;21(3):434–9. https://doi.org/10.1093/europace/euy154.
Errahmouni A, Bun SS, Latcu DG, Saoudi N. Ultrasound-guided venous puncture in electrophysiological procedures: a safe method, rapidly learned: ULTRASOUND-GUIDED VENOUS PUNCTURE IN EP PROCEDURES. Pacing Clin Electrophysiol. 2014;37(8):1023–8. https://doi.org/10.1111/pace.12386.
Rashedi S, Tavolinejad H, Kazemian S, Mardani M, Masoudi M, Masoudkabir F, Haghjoo M. Efficacy and safety of same-day discharge after atrial fibrillation ablation: a systematic review and meta-analysis. Clin Cardiol. 2022;45(2):162–72. https://doi.org/10.1002/clc.23778.
Natale A, Mohanty S, Liu PY, Mittal S, Al-Ahmad A, De Lurgio DB, Horton R, Spear W, Bailey S, Bunch J, Musat D, O’Neill P, Compton S, Turakhia MP. Venous vascular closure system versus manual compression following multiple access electrophysiology procedures: the AMBULATE Trial. JACC Clin Electrophysiol. 2020;6(1):111–24. https://doi.org/10.1016/j.jacep.2019.08.013.
Eldadah ZA, Al-Ahmad A, Bunch TJ, Delurgio DB, Doshi RN, Hook BG, Hranitzky PM, Joyner CA, Mittal S, Porterfield C, Sanchez JE, Thambidorai SK, Wazni OM, McElderry HT. Same-day discharge following catheter ablation and venous closure with VASCADE MVP: A postmarket registry. J Cardiovasc Electrophysiol. 2023;34(2):348–55. https://doi.org/10.1111/jce.15763.
Steinberg BA, Woolley S, Li H, Crawford C, Groh CA, Navaravong L, Ranjan R, Zenger B, Zhang Y, Bunch TJ. Patient-reported outcomes and costs associated with vascular closure and same-day discharge following atrial fibrillation ablation. J Cardiovasc Electrophysiol. 2022;33(8):1737–44. https://doi.org/10.1111/jce.15555.
Sharma PS, Padala SK, Gunda S, Koneru JN, Ellenbogen KA. Vascular complications during catheter ablation of cardiac arrhythmias: a comparison between vascular ultrasound guided access and conventional vascular access: ultrasound guided vascular access for electrophysiologic procedures. J Cardiovasc Electrophysiol. 2016;27(10):1160–6. https://doi.org/10.1111/jce.13042.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
Approval was obtained from the ethics committee of the State Medical Association Hessen (Nr. 2022–2956-evBO). The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Consent to participate
Written informed consent was obtained from all individual participants in the prospective US group of the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Krimphoff, A., Urbanek, L., Bordignon, S. et al. The impact of ultrasound-guided vascular access for catheter ablation of left atrial arrhythmias in a high-volume centre. J Interv Card Electrophysiol (2024). https://doi.org/10.1007/s10840-024-01779-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10840-024-01779-x