Abstract
Background
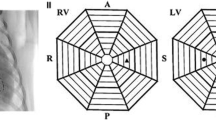
While the triggers for ventricular fibrillation (VF) are well-known, the substrate required for its maintenance remains elusive. We have previously demonstrated dynamic spatiotemporal changes across VF from electrical induction of VF to asystole. Those data suggested that VF drivers seemed to reside in the distal RV and LV. However, signals from these areas were not recorded continuously. The aim of this study was to map these regions of significance with stationary basket electrodes from induction to asystole to provide further insights into the critical substrate for VF rhythm sustenance in canines.
Methods
In six healthy canines, three multipolar basket catheters were positioned in the distal right ventricle (RV), RV outflow tract, and distal left ventricle (LV), and remained in place throughout the study. VF was induced via direct current application from an electrophysiologic catheter. Surface and intracardiac electrograms were recorded simultaneously and continuously from baseline, throughout VF, and until asystole, in order to get a complete electrophysiologic analysis of VF. Focused data analysis was also performed via two defined stages of VF: early VF (immediately after induction of VF to 10 min) and late VF (after 10 min up to VF termination and asystole).
Results
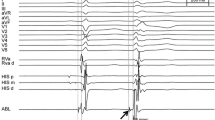
VF was continuously mapped for a mean duration of 54 ± 9 min (range 42–70 min). Immediately after initiation of VF in the early phase, the distal LV region appeared to drive the maintenance of VF. Towards the terminal stage of VF, the distal RV region appeared to be responsible for VF persistence. In all canines, we noted local termination of VF in the LV, while VF on surface ECG continued; conversely, subsequent spontaneous termination of VF in the RV was associated with termination of VF on surface ECG into a ventricular escape rhythm. Continuous mapping of VF showed trends towards an increase in peak-to-peak ventricular electrogram cycle length (p = 0.06) and a decrease in the ventricular electrogram amplitude (p = 0.06) after 40 min. Once we could no longer discern surface QRS activity, we demonstrated local ventricular myocardial capture in both the RV and LV but could not reinitiate sustained VF despite aggressive ventricular burst pacing.
Conclusions
This study describes the evolution of VF from electrical initiation to spontaneous VF termination without hemodynamic support in healthy canines. These data are hypothesis-generating and suggest that critical substrate for VF maintenance may reside in both the distal RV and LV depending on stage of VF. Further studies are needed to replicate these findings with hemodynamic support and to translate such findings into clinical practice.
Graphical abstract
Ventricular fibrillation maintenance may be dependent on critical structures in the distal RV. ECG: electrocardiogram; LV: left ventricle; RV: right ventricle; RVOT: right ventricular outflow tract; VF: ventricular fibrillation





Similar content being viewed by others
Data availability
The data that support the findings of this paper are available from the corresponding author, [CVD], upon reasonable request.
Abbreviations
- CL:
-
Cycle length
- EAM:
-
Electroanatomic mapping
- ECG:
-
Electrocardiogram
- EGM:
-
Electrogram
- EP:
-
Electrophysiology
- HPS:
-
His-Purkinje system
- ICD:
-
Implantable cardioverter-defibrillator
- LV:
-
Left ventricle
- PTSD:
-
Post-traumatic stress disorder
- PVC:
-
Premature ventricular contraction
- RI:
-
Regularity index
- RV:
-
Right ventricle
- RVOT:
-
Right ventricular outflow tract
- SCD:
-
Sudden cardiac death
- VF:
-
Ventricular fibrillation
References
Kong MH, Fonarow GC, Peterson ED, et al. Systematic review of the incidence of sudden cardiac death in the United States. J Am Coll Cardiol. 2011;57:794–801.
Zheng ZJ, Croft JB, Giles WH, Mensah GA. Sudden cardiac death in the United States, 1989 to 1998. Circulation. 2001;104:2158–63.
Fishman GI, Chugh SS, Dimarco JP, et al. Sudden cardiac death prediction and prevention: report from a National Heart, Lung, and Blood Institute and Heart Rhythm Society Workshop. Circulation. 2010;122:2335–48.
Tang PT, Shenasa M, Boyle NG. Ventricular arrhythmias and sudden cardiac death. Card Electrophysiol Clin. 2017;9:693–708.
Haïssaguerre M, Shah DC, Jaïs P, et al. Role of Purkinje conducting system in triggering of idiopathic ventricular fibrillation. Lancet. 2002;359:677–8.
Haïssaguerre M, Shoda M, Jaïs P, et al. Mapping and ablation of idiopathic ventricular fibrillation. Circulation. 2022;106:962–7.
Tan NY, Christopoulos G, Ladas TP, et al. Regional and temporal variation of ventricular and conduction tissue activity during ventricular fibrillation in canines. Circ Arrhythm Electrophysiol. 2021;14:e010281.
Tri J, Asirvatham R, DeSimone CV, et al. Intramural conduction system gradients and electrogram regularity during ventricular fibrillation. Indian Pacing Electrophysiol J. 2018;18:195–200.
Krummen DE, Hayase J, Vampola SP, et al. Modifying ventricular fibrillation by targeted rotor substrate ablation: proof-of-concept from experimental studies to clinical VF. J Cardiovasc Electrophysiol. 2015;26:1117–26.
Liang X, Evans SM, Sun Y. Insights into cardiac conduction system formation provided by HCN4 expression. Trends Cardiovasc Med. 2015;25:1–9.
Kim YH, Xie F, Yashima M, et al. Role of papillary muscle in the generation and maintenance of reentry during ventricular tachycardia and fibrillation in isolated swine right ventricle. Circulation. 1999;100:1450–9.
Boyden PA, Hirose M, Dun W. Cardiac Purkinje cells. Heart Rhythm. 2010;7:127–35.
Haïssaguerre M, Duchateau J, Dubois R, et al. Idiopathic ventricular fibrillation: role of Purkinje system and microstructural myocardial abnormalities. JACC Clin Electrophysiol. 2020;6:591–608.
Berenfeld O, Jalife J. Purkinje-muscle reentry as a mechanism of polymorphic ventricular arrhythmias in a 3-dimensional model of the ventricles. Circ Res. 1998;82:1063–77.
Haissaguerre M, Cheniti G, Hocini M, et al. Purkinje network and myocardial substrate at the onset of human ventricular fibrillation: implications for catheter ablation. Eur Heart J. 2022;43:1234–47.
Imnadze G, Zerm T. Prevention of ventricular fibrillation through de-networking of the Purkinje system: proof-of-concept paper on the substrate modification of the Purkinje network. Pacing Clin Electrophysiol. 2019;42:1285–90.
Livia C, Sugrue A, Witt T, et al. Elimination of Purkinje fibers by electroporation reduces ventricular fibrillation vulnerability. J Am Heart Assoc. 2018;7:e009070.
Dosdall DJ, Tabereaux PB, Kim JJ, et al. Chemical ablation of the Purkinje system causes early termination and activation rate slowing of long-duration ventricular fibrillation in dogs. Am J Physiol Heart Circ Physiol. 2008;295:H883–9.
DeSimone CV, Asirvatham SJ. Purkinje tissue modification and ventricular fibrillation. Pacing Clin Electrophysiol. 2019;42:1291–3.
Bagdonas AA, Stuckey JH, Piera J. Amer NS, Hoffman BF. Effects of ischemia and hypoxia on the specialized conducting system of the canine heart. Am Heart J. 1961;61:206–18.
Friedman PL, Stewart JR, Fenoglio JJ Jr, Wit AL. Survival of subendocardial Purkinje fibers after extensive myocardial infarction in dogs. Circ Res. 1973;33:597–611.
Marrouche NF, Verma A, Wazni O, et al. Mode of initiation and ablation of ventricular fibrillation storms in patients with ischemic cardiomyopathy. J Am Coll Cardiol. 2004;43:1715–20.
Santoro F, Di Biase L, Hranitzky P, et al. Ventricular fibrillation triggered by PVCs from papillary muscles: clinical features and ablation. J Cardiovasc Electrophysiol. 2014;25:1158–64.
Van Herendael H, Zado ES, Haqqani H, et al. Catheter ablation of ventricular fibrillation: importance of left ventricular outflow tract and papillary muscle triggers. Heart Rhythm. 2014;11:566–73.
Sadek MM, Benhayon D, Sureddi R, et al. Idiopathic ventricular arrhythmias originating from the moderator band: electrocardiographic characteristics and treatment by catheter ablation. Heart Rhythm. 2015;12:67–75.
Haïssaguerre M, Hocini M, Cheniti G, et al. Localized structural alterations underlying a subset of unexplained sudden cardiac death. Circ Arrhythm Electrophysiol. 2018;11:e006120.
Oesterlein T, Frisch D, Loewe A, et al. Basket-type catheters: diagnostic pitfalls caused by deformation and limited coverage. Biomed Res Int. 2016;2016:5340574.
Narayan SM, Krummen DE, Rappel WJ. Clinical mapping approach to diagnose electrical rotors and focal impulse sources for human atrial fibrillation. J Cardiovasc Electrophysiol. 2012;23:447–54.
Acknowledgements
We would like to thank Renee Taubel and the veterinary staff of the Mayo Cardiovascular Innovations Laboratory for their contribution to conducting the animal experiments and Cory Scheuermann and Jamie Bush for their help with Rhythmia mapping.
Funding
This work was supported by the Earl A. Wood Career Development Benefactor Award to Dr. Christopher V. DeSimone, MD, PhD.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This study was approved by the Mayo Clinic IACUC committee.
Conflict of interest
CVD, CJM, and SJA report patent filing for intellectual property related to novel tools and methods for ventricular fibrillation mapping and ablation.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ezzeddine, F.M., Ward, R.C., Jiang, Z. et al. Novel insights into the substrate involved in maintenance of ventricular fibrillation: results from continuous multipolar mapping in a canine model. J Interv Card Electrophysiol (2022). https://doi.org/10.1007/s10840-022-01333-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10840-022-01333-7