Abstract
Background
Since the CRYSTAL-AF trial, implantation and usage of implantable loop recorder (ICM) after cryptogenic stroke (CS) for detection of atrial fibrillation (AF) has increased. However, it is unclear which CS patients would most benefit from long term ICM monitoring. This study aims to determine the risk factors in patients that would confer maximum benefit from ICM placement following CS.
Methods
A Columbia University Institutional Review Board (IRB) approved retrospective analysis of medical records of 125 patients with CS followed by implantation of ICM was evaluated. Univariable and multivariable time-to-event analyses were performed on demographics, hours of activity and variability (HRV), stroke location, thrombosis etiology, and CHA2DS2 − VASc score. The primary outcome was presence of ICM-detected AF defined as AF lasting at least 2 min.
Results
One hundred twenty-five patients (mean 67.6 years ± 2.4 years, 60% male) were followed for at least 3 months. Twenty-two patients (18%) were found to have clinically verified detected AF; median of time to detection was 95 days. Upon univariable demographic analysis followed by multivariable Cox regression analysis, individuals with age 75 or older (HR: 3.987, p = 0.0046) or LVEF 40% and lower (HR: 3.056, p = 0.0213) had significantly higher risk of AF. Diabetics also had a lower AF detection in multivariable analysis (HR: 0.128, p = 0.0466).
Conclusions
Age 75 or older and LVEF ≤40% were the factors on multivariable analysis that predicted AF detection. Diabetes is a possible significant factor which should be evaluated further. CHA2DS2 − VASc score was notably not predictive of AF detected on ICM.
Similar content being viewed by others
1 Introduction
The use of remote digital monitoring has increased during the COVID-19 pandemic, with a newfound importance placed on technology which can allow for patients to be observed from afar [1]. ICMs are a remote monitoring solution which can give a tremendous amount of information to physicians without seeing patients in person and will likely only have added importance in the post-pandemic era. Features which would be especially important in this type of digital monitoring would be rhythm, heart rate, hours of activity, and HRV, especially given the ability of these metrics to measure changes to cardiac homeostasis [2,3,4,5]. It remains uncertain how useful these specific features are for detecting AF.
Cerebral vascular accident (CVA) and transient ischemic attacks (TIA) are two of the leading causes of mortality worldwide. Despite widespread public health success in the field, the causes of all types of CVA and TIA are not fully understood [6]. In 20–40% of patients with stroke, it is difficult to determine a clear etiology after routine evaluation, leading to classification of the stroke as CS [7]. As one in six strokes can be traced to AF, with rates as high as 36% for those over the age of 80, undiagnosed AF remains a prime suspect cause of CS [8]. Identifying underlying AF on extended cardiac monitoring suggests cardio-embolic etiology and often prompts anticoagulation to reduce risk of recurrent CVA [9].
The landmark CRYSTAL-AF trial showed a sixfold or higher increase in detection of AF with ICM as compared to traditional 24-h Holter monitoring [10]. The results of this trial lead to ICM implantation following CS to become widespread, as evidenced by the increasing number of ICMs implanted at the Columbia University Medical Center between 2013 and 2017. Guidelines following this landmark study have been modified to reflect this added importance of cardiac monitoring following CS [11]. However, uncertainty regarding the economic value and appropriate usage duration of ICM cardiac monitoring post-CS still persists. ICM monitoring has been noted to have a considerable cost associated with it, which represents a significant road-block in its wider distribution and underscores the need for a deeper understanding of which patients would benefit the most [12]. Although an ICM is easy to place and there is minimal risk, more research is needed to determine which patients would benefit the most from longer-term ICM monitoring and what digital health information from monitor may be used to predict occurrence of AF.
2 Methods
2.1 Patient population and data elements
We conducted a retrospective chart review study of all patients with ICM placed at the Columbia University Medical Center from 2013 to 2017 for indication of CS or TIA with follow-up of up to 36 months to determine the characteristics of patients who most benefit from ICM placement and time to AF detection after ICM placement with IRB approval obtained prior to the start of the study. We obtained several baseline characteristics for each patient including age, race, components of CHA2DS2 − VASc score, and medications. Furthermore, we recorded imaging reports for each patient including CT head, MRI brain, MRA head and neck, echocardiogram, and EKG. ICM data collected included minimum heart rate, maximum heart rate, hours of activity, HRV, duration of longest AF episode, number of AF episodes recorded, and whether or not there were reported symptoms.
For patients to be included in our study, eligibility requirements included placement of ICM for indication of cryptogenic CVA or TIA. We verified indication for ICM both in ICM reports and in corresponding clinical documentation. Some patients were referred for ICM placement without the index CVA or TIA at CUMC. Therefore, we deferred classification of cryptogenic CVA/TIA to the medical providers evaluating each index event. Patients were required to have at least 3 months of follow-up after ICM placement to be included in our study. Patients were also required to have no longer than 3 years, or 36 months, of ICM duration to account for device battery life. Furthermore, AF events were required to be at least 2 min of duration, within the bounds of LINQ ICM’s detection capabilities. These episodes were only included in the study if corresponding clinical documentation confirmed that episodes in ICM reports were consistent with AF after review of rhythm strips. All patients were implanted with Medtronic Reveal LINQ ICMs. HRV was determined by the device, by measuring each atrial interval or ventricular interval and calculating the median atrial/ventricular interval every 5 min which was then plotted as a variability value in milliseconds for each day. Data from the ICM was obtained through remote monitoring. When an AF episode was detected, the first 2 min of ECG from that episode was stored in the device. The longest detected AF episode ≥10 min in duration is preserved in the ICM memory. In addition, several parameters where used for alerts: bradycardia ~30 bpm for 3 s, asystole for 3 s, ventricular tachycardia 16 beats at appropriate rate based on age.
2.2 Statistical analysis
After completion of chart review, univariable and multivariable analyses were performed using both Excel and SAS Software (SAS Studio Release 3.8, Cary, North Carolina). Baseline characteristics were assessed comparing patients with and without AF reported by ICM, with chi-square and t tests being performed to compare the two groups. Time-to-event analyses were performed using the Kaplan-Meier method. Multivariable time-to-event analyses included age, sex, and other variables with a p value < 0.20 in univariable analyses. Statistical tests were run with a two-sided alpha-value of 0.05.
3 Results
A total of 478 ICM devices were placed at the Columbia University Medical Center between 2013 and 2017. Of these, 154 were placed for indication of cryptogenic stroke or TIA, and 125 of these 154 patients had between 3 and 36 months of follow-up (Fig. 1). Of these 125 patients, 22 patients had ICM-reported AF over the follow-up period. Average time to ICM implant post-stroke was 821 days for the entire cohort and was not significantly different between patients with AF detected and those without (168 vs. 971 days, p = 0.52).
Baseline characteristics for patients are seen in Table 1. The mean age of patients was 67.6 years, and 60% of patients were men. The mean CHA2DS2 − VASc score prior to index event was 3.12 for all patients. Within the study group of 130 patients, only 22 (18%) were found to have verified episodes of AF. On average, the ICMs were in place for 492 days with a range from 94 to 1094 days or approximately 3 years. ICMs remained in place for a longer amount of time in patients where AF was detected compared to those where AF was not detected (646 days vs. 460 days, p = 0.01). Of demographic variables observed in Table 1, age, sex, LVEF, diabetes, and BMI all had p values <0.20 and were included in subsequent multivariable Cox regression analyses. Of these variables, age, sex, and diabetes had p values <0.05, reflecting significant differences between patients with and without detected AF.
The duration of verified AF episodes ranged from 2 to 1440 min (24 h), with a median duration of 120 min and an average duration of 174.5 min. Hours of activity and HRV were not significantly different between patients with or without verified detected AF (Table 2). Furthermore, there were no significant differences in the maximum number of daily activity hours of the ICM between patients with or without verified detected AF.
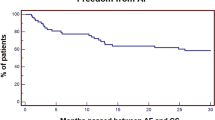
The median time to verified AF detection was 95 days, or approximately 3 months, and ranged from 3 days to 3 years (Fig. 2). Furthermore, there was an increasing cumulative rate of AF detection over the study period, however, of the entire patient cohort 50% of the AF was detected within the first 250 days of follow-up.
Time-to-event analyses analyzing time to verified AF detection showed discrepancies between age group categories (Fig. 3). Patients were divided into age groups less than 75 years old (n = 81) and greater or equal to 75 years old (n = 44). These group cut-offs correspond to the same 75-year-old cut-off used in calculating CHA2DS2 − VASc score. The mean time to AF detection in age groups less than 75 years and greater than or equal to 75 years, respectively, were 447 and 403 days, reflecting earlier AF detection in older individuals. The longest time to AF detection in the age group less than 75 years was 795 days compared to 559 days in the 75 or older age group.
Multivariable Cox regression analysis showed a statistically significant difference between these two age groups, with individuals 75 or older having a statistically higher risk of AF compared to those less than 75 (HR = 3.987, p = 0.0046, Table 3). Furthermore, of included variables shown in Table 3 multivariable analysis, LVEF ≤40% (HR = 3.056, p = 0.0213) showed statistically greater risk of AF compared to those with LVEF > 40%. Interestingly, patients with diabetes had a statistically lower risk of AF (HR = 0.128, p = 0.0466) than those without when evaluated in a multivariable model. Sex and BMI both did not yield significant differences in AF-likelihood when observed in the multivariable analysis.
4 Discussion
Through this retrospective chart review of patients placed with an ICM at the Columbia University between 2013 and 2017, we find several significant findings which can impact future clinical practice and how remote monitoring is used in an increasingly distanced care setting, especially given the significant cost associated with such monitoring. AF was reported in the ICMs of 22 patients (18%) over the 36-month follow-up period. Therefore, a significant percent of the cohort had detected and verified AF, underscoring the high rate of AF in this population. Furthermore, it seems as though the first 250 days of monitoring are especially important in detecting AF as 50% of the ultimate verified AF was detected by ICM within this initial interval. Current American Heart Association guidelines call for cardiac rhythm monitoring for up to 30 days in cryptogenic stroke patients. Data from this study supports ICM monitoring > 30 days, offering possible evidence that this 30-day monitoring period could be extended to a greater than 30-day monitoring suggestion to detect more AF in these patients. Furthermore, this finding further underscores the overall importance of using remote monitoring for patients with cryptogenic stroke indications, as a considerable cohort of these patients had detected AF.
In terms of predictive metrics, the findings of the study reveal that age, LVEF, and diabetes status were all predictive metrics of AF detected by ICM. Interestingly, individuals aged 75 and older had a significantly greater risk of AF compared to those less than 75, even when included in a multi-variable context. This result suggests increased importance of remote monitoring specifically for the age group 75 and older. We chose to dichotomize age in our analysis to investigate what age became a significant risk factor of AF detection.
Furthermore, multivariable findings also found similarly statistically significant greater AF risk in patients with LVEF ≤ 40% or without diabetes. It is important to note, however, that only 26 patients of the overall 125 (21%) were diagnosed with diabetes, of which only 1 patient had reported AF. Therefore, due to the small sample size of individuals with both diabetes and AF, it is difficult to conclude a relationship between the two variables and further studies should be conducted to evaluate this finding on larger samples. Analyses from the CRYSTAL-AF trial found similar predictive capabilities of age and diabetes, but unlike our study, also found CHADS2 score to be a predictive indicator of AF [13]. Although meeting the threshold for being included in the multivariable analysis from the univariate analyses, sex and BMI did not yield significant findings, despite their traditional importance in recognizing patients at risk of AF. It is important to note that the findings of the multivariable analysis can stand alone; therefore, these significant findings suggest that LVEF and age (and possibly diabetes, however, its smaller sample size is a possible limitation) are strong metrics that may inform higher priority for certain patients to be implanted with ICMs for AF monitoring following CS, likely for a period 100 days or longer.
The CRYSTAL-AF trial results reflected a median time to AF detection of 84 days. The results of this study were consistent with the results of the CRYSTAL-AF trial, finding a median time of 95 days [10]. Furthermore, the rate of detection of AF in the CRYSTAL-AF trial was consistent with that in this study after 12 months of follow-up, with 12.4% of patients in the CRYSTAL-AF trial having detected AF (versus 9% in our study). At the 36-month follow-up time period, however, the CRYSTAL-AF trial detected AF in roughly 30% of patients compared to just 18% in our study. This difference could possibly be due to differences in assessments of patients between the two studies before diagnosis of cryptogenic TIA. Other observational studies have shown detection rates of AF higher than this study, showing rates upwards of 25% using ICMs [14].
In this study of real-world implantation of ICMs and utilization of remote monitoring resources, we show that patients of a higher age group derive the most benefit from remote monitoring. This study is also novel in that it is the first study to study clinical data regarding HRV and hours of activity for patients following CS [15,16,17,18,19,20,21]. These two factors were monitored in our analysis as they were hypothesized to be important prognostic variables. However, they were not statistically significant predictors of AF.
5 Limitations
This study has a few possible limitations which may impact study results. As this was a retrospective study, it is important to note that associations found do not imply causal relationships. Given there was no fixed criteria for assessment of CVA/TIA and this was left up to the interpretation of clinicians, misclassification bias could impact which patients were ultimately included in the study. This was also a retrospective chart analysis, limiting certain aspects of follow-up which may have detected more AF. A considerable number of patients in this study also had history of prior CVA/TIA (35%), raising the possibility that they may have had previously undiagnosed AF that could potentially give rise to stroke. The rate of AF detection in CVA patients in our study was 17% and the rate of AF detection in TIA patients was 20%. Our statistical test for differences between AF detection between these two groups yielded a p value of 0.72, and so, we concluded that there did not appear to be a significant difference in our study between CVA and TIA index events and AF detection. However, given that we had significantly more CVA patients than TIA patients, further studies on larger subsets could possibly explore this further.
While there was no statistically significant difference in time from index event to device placement between the groups, the average was high at 821 days for the group at a whole with a very large standard deviation of 920 days. This also raises the possibility that certain patients, especially those with longer follow-up times, may have had AF during the window between index event and device placement, and is a limitation of this study. This finding could therefore potentially explain part of the high detection rate within the first 250 days of the study in which 50% of eventual AF was discovered. Furthermore, there were very few patients less than 40 years of age (5% of overall cohort), none of whom had AF detected. Likewise, few study participants (6% of overall cohort) had a CHA2DS2 − VASc score of 0. These relatively low amounts of patients falling into these categories may possibly impact the predictive ability of these variables in regression analyses. The average follow-up time for patients in this retrospective study was 477 days, which although is long may not have been long enough to catch an arrhythmic episode causing stroke. Unfortunately, 25% of the patients were lost to follow-up which limited our longer term follow-up. Further studies should be performed on larger cohorts to test the viability of both age and CHA2DS2 − VASc score in terms of predictive ability for AF to ascertain if there is a lower age limit which confers a low-risk for whom remote monitoring may not be necessary.
6 Conclusion
Remote monitoring will have increased importance in the era following the SARS-CoV-2 pandemic. HRV and hours of activity information are both added strengths of remote monitoring, however, neither were predictive of AF in our analysis despite their clinical value. Multivariable Cox Regression analysis found that individuals 75 or older had a significantly greater risk of AF. LVEF also shows significant potential predictive capabilities on its own in multivariable models, with significantly greater risk of AF for patients with LVEF 40% or lower. Diabetes also shows promise as a predictive tool of AF; however, further studies must be conducted to further substantiate this hypothesis. While sex and BMI are important variables in estimating stroke risk, neither is significantly predictive for AF following CS in multivariable models. Overall, roughly 18% of patients followed between 2013 and 2017 at CUMC following cryptogenic stroke had clinician-verified detected AF, reflecting the high rate of AF in this population. This study also suggests that clinicians should continue to employ remote monitoring for a period of at least 30 days following cryptogenic stroke, with evidence supporting longer follow-up periods, especially those at higher-risk spectrums of age and LVEF.
References
Portnoy J, Waller M, Elliott T. Telemedicine in the Era of COVID-19. J Allergy Clin Immunol Pract. 2020;8:1489–91.
Nenna A, et al. Heart rate variability: a new tool to predict complications in adult cardiac surgery. J Geriatr Cardiol. 2017;14:662–8.
Lees T, et al. Heart rate variability as a biomarker for predicting stroke, post-stroke complications and functionality. Biomark Insights. 2018;13:1177271918786931.
Khan AA, Lip GYH, Shantsila A. Heart rate variability in atrial fibrillation: the balance between sympathetic and parasympathetic nervous system. Eur J Clin Investig. 2019;49:e13174.
Shaffer F, Ginsberg JP. An overview of heart rate variability metrics and norms. Front Public Health. 2017;5:258.
Lackland DT, et al. Factors influencing the decline in stroke mortality: a statement from the American Heart Association/American Stroke Association. Stroke. 2014;45:315–53.
Bal S, et al. High rate of magnetic resonance imaging stroke recurrence in cryptogenic transient ischemic attack and minor stroke patients. Stroke. 2012;43:3387–8.
Hart RG, et al. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol. 2014;13:429–38.
Reiffel JA. Atrial fibrillation and stroke: epidemiology. Am J Med. 2014;127:e15–6.
Sanna T, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370:2478–86.
Albers GW, et al. Heart rhythm monitoring strategies for cryptogenic stroke: 2015 Diagnostics and Monitoring Stroke Focus Group Report. J Am Heart Assoc. 2016;5:e002944.
Krahn AD, Klein GJ, Yee R, Manda V. The high cost of syncope: cost implications of a new insertable loop recorder in the investigation of recurrent syncope. Am Heart J. 1999;137:870–7.
Thijs VN, et al. Predictors for atrial fibrillation detection after cryptogenic stroke: results from CRYSTAL AF. Neurology. 2016;86:261–9.
Cotter PE, et al. Incidence of atrial fibrillation detected by implantable loop recorders in unexplained stroke. Neurology. 2013;80:1546–50.
Gladstone DJ, et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med. 2014;370:2467–77.
Petrovicova A, et al. Detection of occult paroxysmal atrial fibrilation by implantable long-term electrocardiographic monitoring in cryptogenic stroke and transient ischemic attack population: a study protocol for prospective matched cohort study. BMC Cardiovasc Disord. 2015;15:160.
Wang G, Joo H, Tong X, George MG. Hospital costs associated with atrial fibrillation for patients with ischemic stroke aged 18-64 years in the United States. Stroke. 2015;46:1314–20.
Dalen JE, Alpert JS. Silent atrial fibrillation and cryptogenic strokes. Am J Med. 2017;130:264–7.
Carmona-Puerta R, Castro-Torres Y. Atrial fibrillation and cryptogenic stroke. What is the current evidence? Role of electrocardiographic monitoring. J Arrhythm. 2018;34:1–3.
Haeusler KG, Tutuncu S, Schnabel RB. Detection of atrial fibrillation in cryptogenic stroke. Curr Neurol Neurosci Rep. 2018;18:66.
Milstein NS, Musat DL, Allred J, Seiler A, Pimienta J, Oliveros S, Bhatt AG, Preminger M, Sichrovsky T, Shaw RE, Mittal S. Detection of atrial fibrillation using an implantable loop recorder following cryptogenic stroke: implications for post-stroke electrocardiographic monitoring. J Interv Card Electrophysiol. 2020;57(1):141–147. https://doi.org/10.1007/s10840-019-00628-6.
Acknowledgements
The authors have no relevant disclosures.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This study conducted a retrospective chart review analysis involving human participants. This study was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The Human Investigation Committee (IRB) of Columbia University approved this study.
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 95 kb)
Rights and permissions
About this article
Cite this article
Desai, A.D., Howe, E., Coromilas, E. et al. Predictors of atrial fibrillation on implantable cardiac monitoring for cryptogenic stroke. J Interv Card Electrophysiol 65, 7–14 (2022). https://doi.org/10.1007/s10840-021-00985-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-021-00985-1