Abstract
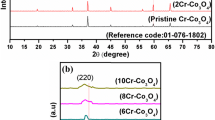
Mixed transition metal oxides have attracted great attention in supercapacitors applications due to their better electrochemical performance than their single oxides. In this work, iron cobaltite (FeCo2O4) and its single metal oxides i.e. iron oxide (Fe2O3) and cobalt oxide (Co3O4) were synthesized by a simple hydrothermal process. The structural, spectroscopic and morphological properties were studied using X-ray diffraction (XRD), Fourier transform infrared (FTIR) spectroscopy and field-emission scanning electron microscope (FESEM). XRD and FTIR results show the composition of the products. The obtained iron oxide was α-Fe2O3. FESEM images show that FeCo2O4 and its single metal oxides exhibit different morphology even though they were synthesized via similar method. The electrochemical properties of the α-Fe2O3, Co3O4 and FeCo2O4 electrodes were examined by cyclic voltammetry (CV), galvanostatic charge/discharge (GCD) and electrochemical impedance spectroscopy (EIS) in a 6 M KOH electrolyte solution. At comparable current density, the FeCo2O4 electrode has the highest specific capacitance (Csp), followed by Co3O4 and α-Fe2O3. An asymmetric FeCo2O4/KOH/GO supercapacitor was fabricated. The supercapacitor exhibits maximum energy density of 14.5 Wh kg−1 and maximum power density of 2177 W kg−1. It demonstrates 60% rate capability after 1000 continuous charge-discharge cycles at 1 A g−1.















Similar content being viewed by others
References
G. Wang, L. Zhang, J. Zhang, Chem. Soc. Rev. 41, 797 (2012)
T. Winie, A.K. Arof, in Nanostructured Polymer Membranes: Application Vol. 2, eds. By P.M. Visakh, O. Nazarenko (Scrivener Publising (Wiley), Beverly, 2016), p. 311
B.G. Hyun, H.J. Son, S. Ji, J. Jang, S.H. Hur, J.U. Park, J. Electroceram. 38, 43 (2017)
A. Annu, B. Bhattacharya, P.K. Singh, P.K. Shukla, H. Rhee, J. Alloys Compd. 691, 970 (2017)
S. Siyahjani, S. Oner, P.K. Singh, High Perform. Polym. 30, 1 (2018)
M. Kunowsky, M. Linares-Solano, A. Garcia-gomez, V. Barranco, J.M. Rojo, J.D. Carruthers, Int. J. Appl. Ceram. Technol. 12, 127 (2015)
S. Ahmed, A. Ahmed, M. Rafat, J. Saudi Chem. Soc. 22, 993 (2018)
S. Najib, E. Erdem, Nanoscale Adv. 20, 12817 (2019)
S. Dhibar, C.K. Das, J. Appl. Polym. Sci. 134, 44724 (2017)
L. Wang, C. Zhang, X. Jiao, Z. Yuan, Nano Res. 12, 1129 (2019)
A. Eftekhari, L. Li, Y. Yang, J. Power Sources 347, 86 (2017)
Y. Xie, D. Wang, J. Ji, Energy Technol. 4, 714 (2016)
M. Rajesh, C.J. Raj, R. Manikandan, B. Chul, S. Yeup, K. Hyun, Today Energy 6, 96 (2017)
K. Kan, L. Wang, P. Yu, W. Zhou, R. Wang, Y. Lin, K. Shi, H. Fu, Chempluschem. 81, 242 (2016)
X. Liu, F. Liu, Eur. J. Inorg. Chem. 2018, 987 (2018)
J. Sodtipinta, H.K. Kim, S.W. Lee, S.M. Smith, P. Pakawatpanurut, K.B. Kim, J. Electroceram. 35, 111 (2015)
T. Nesakumar, J. Immanuel, R. Atchudan, Y.R. Lee, Int. J. Hydrog. Energy 44, 2323 (2018)
Y. Li, X. Han, T. Yi, Y. He, X. Li, J. Energy Chem. 31, 54 (2019)
M. Shanmugavadivel, V.V. Dhayabaran, M. Subramanian, J. Phys. Chem. Solids 133, 15 (2019)
Q. Gao, J. Wang, J. Wang, J. Alloys Compd. 789, 193 (2019)
S. Liu, D. Ni, H. Li, K.N. Hui, C. Ouyang, S.C. Jun, J. Mater. Chem. A 6, 10674 (2018)
M. Liu, L. Kong, C. Lu, X. Li, Y. Luo, L. Kang, ACS Appl. Mater. Interfaces 4, 4631 (2012)
M.R. Joya, J. Baron-jaimez, J. Barba-ortega, J. Phys.: Conf. Ser. (2013). https://doi.org/10.1088/1742-6596/466/1/012004
S. Yu, V.M.H. Ng, F. Wang, Z. Xiao, C. Li, L.B. Kong, W. Que, K. Zhou, J. Mater. Chem. A 6, 9332 (2018)
H. Nan, L. Yu, W. Ma, B. Geng, X. Zhang, Dalt. Trans. 44, 9581 (2015)
Z. Chen, C.X. Kronawitter, B.E. Koel, Phys. Chem. Chem. Phys. 17, 29387 (2015)
H. Zhang, C. Lu, H. Hou, Y. Ma, S. Yuan, J. Alloys Compd. 797, 970 (2019)
X. Liu, H. Wang, C. Su, P. Zhang, J. Bai, J. Colloid Interface Sci. 351, 427 (2010)
B. Li, Y. Xie, C. Wu, Z. Li, J. Zhang, Mater. Chem. Phys. 99, 479 (2006)
D.C. Marcano, D.V. Kosynkin, J.M. Berlin, A. Sinitskii, Z. Sun, A. Slesarev, L.B. Alemany, W. Lu, J.M. Tour, ACS Nano 4, 4806 (2010)
S. Roldán, D. Barreda, M. Granda, R. Menéndez, R. Santamaría, C. Blanco, Phys. Chem. Chem. Phys. 17, 1084 (2015)
D.P. Dubal, P. Gómez-Romero, B.R. Sankapal, R. Holze, Nano Energy 11, 377 (2015)
J. Wang, Y. Yang, Z. Huang, F. Kang, Carbon 61, 190 (2019)
L. Zhang, H. Li, K. Li, L. Li, J. Wei, L. Feng, Q. Fu, J. Alloys Compd. 680, 146 (2016)
C. Zhu, C. Chen, L. Hao, Y. Hu, Z. Chen, Solid State Commun. 130, 681 (2004)
M. Behrens, F. Girgsdies, Zeitschrift für Anorg und Allg Chemie 636, 919 (2010)
F. Karadas, C.T. Yavuz, S. Zul, S. Aparicio, G.D. Stucky, M. Atilhan, Langmuir 27, 10642 (2011)
G. Zhu, C. Xi, M. Shen, C. Bao, J. Zhu, ACS Appl. Mater. Interfaces 6, 17208 (2014)
S.G. Mohamed, S.Y. Attia, H.H. Hassan, Microporous Mesoporous Mater. 251, 26 (2017)
G. Xu, Z. Zhang, X. Qi, X. Ren, S. Liu, Q. Chen, Z. Huang, J. Zhong, Ceram. Int. 44, 120 (2018)
Q. Gao, J. Wang, B. Ke, J. Wang, Y. Li, Ceram. Int. 44, 18770 (2018)
M. Su, C. He, K. Shih, Ceram. Int. 42, 14793 (2016)
X. Zhou, Y. Zhong, M. Yang, Q. Zhang, J. Wei, Z. Zhou, ACS Appl. Mater. Interfaces 7, 12022 (2015)
X. Leng, L. Wu, Y. Liu, C. Li, S. Wei, Z. Jiang, G. Wang, J. Lian, Q. Jiang, J. Mater. Chem. A 4, 17171 (2016)
J. Xu, P. Gao, T.S. Zhao, Energy Environ. Sci. 5, 5333 (2012)
L.S. Lobo, S. Kalainathan, A.R. Kumar, Superlattice. Microst. 88, 116 (2015)
M.M. Kadam, R. Lokare, K.M.V.K. Kireeti, V.G. Gaikar, N.I. Jha, RSC Adv. 4, 62737 (2014)
S. Perumbilavil, P. Sankar, T.P. Rose, R. Philip, Appl. Phys. Lett. (2015). https://doi.org/10.1063/1.4928124
B. Dehghanzad, M.K.R. Aghjeh, O. Rafeie, A. Tavakoli, A.J. Oskooie, RSC Adv. 4, 62737 (2015)
H. Gao, J. Xiang, Y. Cao, Nanotechnology 28, 1 (2017)
F. Zhang, C. Yuan, X. Lu, L. Zhang, Q. Che, X. Zhang, J. Power Sources 203, 250 (2012)
T. Winie, A.K. Arof, in Physical Chemistry of Macromolecules, eds. by C.H. Chan, C.H. Chua, S. Thomas (Apple Academic Press, Florida, 2014), p. 335
Z. Huang, Z. Zhang, X. Qi, X. Ren, G. Xu, P. Wan, X. Sun, H. Zhang, Nanoscale 8, 13273 (2016)
Z. Zhang, Y. Liu, Z. Huang, L. Ren, X. Qi, X. Wei, J. Zhong, Phys. Chem. Chem. Phys. 17, 20795 (2015)
S. Khalid, C. Cao, L. Wang, Y. Zhu, Sci. Rep. 6, 1 (2016)
G. He, J. Li, H. Chen, J. Shi, X. Sun, S. Chen, X. Wang, Mater. Lett. 82, 61 (2012)
A. Daraghmeh, S. Hussain, I. Saadeddin, L. Servera, E. Xuriguera, A. Cornet, A. Cirera, Nanoscale Res. Lett. 12, 639 (2017)
B. Zhu, S. Tang, S. Vongehr, H. Xie, J. Zhu, X. Meng, Chem. Commun. 52, 2624 (2016)
N.R. Chodankar, D.P. Dubal, Y. Kwon, D. Kim, NPG Asia Mater. (2017). https://doi.org/10.1038/am.2017.145
Acknowledgements
The authors wish to thank the Ministry of Science and Technology Taiwan and Universiti Teknologi MARA Malaysia for supporting this work through MOSTI07-2221-E-009-130 and 600-IRMI/PERDANA 5/3 BESTARI (040/2018).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Saaid, F.I., Arsyad, A., Azman, N.S.H. et al. The synergistic effect of iron cobaltite compare to its single oxides as cathode in supercapacitor. J Electroceram 44, 183–194 (2020). https://doi.org/10.1007/s10832-020-00209-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10832-020-00209-4