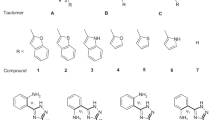
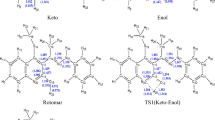
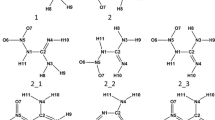
Abstract
Herein, we present a simple and versatile theoretical–experimental approach to assess the tautomeric distribution on 5(6)-aminobenzimidazole (5(6)-ABZ) derivatives in solution via one-photon absorption. The method is based on the optimized weighted sum of the theoretical spectra of the corresponding tautomers. In this article we show how the choice of exchange-correlation functional (XCF) employed in the calculations becomes crucial for the success of the approach. After the systematic analysis of XCFs with different amounts of exact-exchange we found a better performance for B3LYP and PBE0. The direct test of the proposed method on omeprazole, a well-known 5(6)-benzimidazole based pharmacotherapeutic, demonstrate its broader applicability. The proposed approach is expected to find direct applications on the tautomeric analysis of other molecular systems exhibiting similar tautomeric equilibria.
Graphical abstract
Using a weighted sum of the corresponding individual tautomer theoretical spectra, the tautomeric population of benzimidazole derivatives in solution and at room temperature is directly determined through the theoretical–experimental fitting of the UV–Vis spectra of the tautomeric mixture at equilibrium. The reliability of the proposed method is based on the existent spectral difference between the two species.












Similar content being viewed by others
References
Brink NG, Folkers K (1949) J Am Chem Soc 71:2951
Wright JB (1951) Chem Rev 48:397–541
Preston PN (1974) Chem Rev 74:279–314
Bansal Y, Silakari O (2012) Biorg Med Chem 20:6208–6236
Kazimierczuk Z, Andrzejewska M, Kaustova J, Klimesova V (2005) Eur J Med Chem 40:203–208
Richards ML, Lio SC, Sinha A, Tieu KK, Sircar JC (2004) J Med Chem 47:6451–6454
Vijayakumar K, Ahamed AJ (2010) J Chem Pharm Res 2:215–224
Cheng J, Xie J, Luo X (2005) Bioorg Med Chem Lett 15:267–269
Goker H, Ozden S, Yildiz S, Boykin DW (2005) Eur J Med Chem 40:1062–1069
Boufatah N, Gellis A, Maldonado J, Vanelle P (2004) Tetrahedron 60:9131–9137
Brandon DL, Binder RG, Bates AH, Montague WC (1994) J Agric Food Chem 42:1588–1594
Desai KG, Desai KR (2006) Biorg Med Chem 14:8271–8279
Eicher T, Hauptmann S (2003) The chemistry of heterocycles. Wiley-VCH, Weinheim
Katritzky A, Hall CD, El-Gendy B-D, Draghici B (2010) J Comput Aided Mol Des 24:475–484
Strazewski P (1988) Nucl Acids Res 16:9377–9398
Guengerich FP (2006) Chem Rev 106:420–452
Tothadi S, Bhogala BR, Gorantla AR, Thakur TS, Jetti RK, Desiraju GR (2012) Chem Asian J 7:330–342
Cruz-Cabeza AJ, Groom CR (2011) CrystEngComm 13:93–98
Angeles Garcia M, Claramunt RM, Solcan T, Milata V, Alkorta I, Elguero J (2009) Magn Reson Chem 47:100–104
Claramunt RM, Lopez C, Alkorta I, Elguero J, Yang R, Schulman S (2004) Magn Reson Chem 42:712–714
Houben L, Ramaekers R, Adamowicz L, Maes G (2004) Internet Electron J Mol Des 3:163–181
Brown TN, Mora-Diez N (2006) J Phys Chem B 110:9270–9279
Zimmermann AE, Walters JK, Katona BG, Souney PE, Levine D (2001) Clin Ther 23:660–679; discussion 645
Runge E, Gross EKU (1984) Phys Rev Lett 52:997–1000
Dierksen M, Grimme S (2006) J Chem Phys 124:174301
Becke AD (1988) Phys Rev A 38:3098–3100
Becke AD (1993) J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Phys Rev B: Condens Matter 37:785–789
McLean AD, Chandler GS (1980) J Chem Phys 72:5639–5648
Mennucci B, Tomasi J, Cammi R, Cheeseman JR, Frisch MJ, Devlin FJ, Gabriel S, Stephens PJ (2002) J Phys Chem A 106:6102–6113
Tomasi J, Mennucci B, Cammi R (2005) Chem Rev 105:2999–3093
Perdew JP, Ernzerhof M, Burke K (1996) J Chem Phys 105:9982–9985
Adamo C, Barone V (1997) Chem Phys Lett 274:242–250
Zhao Y, Truhlar D (2008) Theor Chem Acc 120:215–241
Yanai T, Tew DP, Handy NC (2004) Chem Phys Lett 393:51–57
Vydrov OA, Scuseria GE (2006) J Chem Phys 125:234109-1–234109-9
Schönherr T (ed) (2004) Optical spectra and chemical bonding in transition metal complexes. Springer, New York
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr. JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam NJ, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian, Inc., Wallingford
Caricato M, Trucks GW, Frisch MJ, Wiberg KB (2010) J Chem Theory Comput 7:456–466
Caricato M, Trucks GW, Frisch MJ, Wiberg KB (2010) J Chem Theory Comput 6:370–383
Jacquemin D, Wathelet V, Perpète EA, Adamo C (2009) J Chem Theory Comput 5:2420–2435
Laurent AD, Jacquemin D (2013) Int J Quantum Chem 113:2019–2039
Jacquemin D, Perpète EA, Scalmani G, Frisch MJ, Ciofini I, Adamo C (2007) Chem Phys Lett 448:3–6
Ohishi H, In Y, Ishida T, Inoue M, Sato F, Okitsu M, Ohno T (1989) Acta Crystallogr Sect C 45:1921–1923
Acknowledgments
This work was partially supported by the National Science Foundation through Grant Number CHE-0840431. The computing time provided by STOKES ARCC is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Diaz, C., Llovera, L., Echevarria, L. et al. Assessment of the tautomeric population of benzimidazole derivatives in solution: a simple and versatile theoretical–experimental approach. J Comput Aided Mol Des 29, 143–154 (2015). https://doi.org/10.1007/s10822-014-9810-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10822-014-9810-7