Abstract
Purpose
To examine the effects of age, mature oocyte number, and cycle number on cumulative live birth rates after planned oocyte cryopreservation (OC), with the goal of developing a patient counselling tool.
Methods
We performed a retrospective cohort study of all patients with ≥ 1 autologous oocyte thaw at our university-affiliated fertility center before 12/31/2023. Patients were included if they (1) had a live birth or ongoing pregnancy > 12 weeks from OC, or (2) used all oocytes and euploid/untested embryos from OC. Primary outcome was cumulative live birth / ongoing pregnancy rate (CLBR).
Results
527 patients with 1 OC cycle, 149 patients with 2 OC cycles, and 55 patients with ≥ 3 OC cycles were included. Overall CLBR was 43%. CLBR was > 70% among patients who thawed ≥ 20 mature oocytes that were cryopreserved at age < 38 years. Multiple logistic regression showed that age at first OC and total number of mature oocytes thawed independently predicted CLBR, but number of OC cycles did not.
Conclusion
Patients must be counselled that younger age at OC and more mature oocytes improve CLBR. However, additional OC cycles do not independently improve CLBR. Our results can help patients decide whether to pursue additional OC cycles to obtain more oocytes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Planned oocyte cryopreservation (OC) is now widely accepted as a fertility preservation method for women facing age-related fertility decline. This technology can result in pregnancy rates comparable with fresh in vitro fertilization (IVF) [1,2,3,4,5,6,7] and appears safe for offspring [2, 8]. A recent report from our center, which reviewed 543 planned OC patients who thawed autologous oocytes, demonstrated that the majority of patients who were < 38 years (y) at OC or who thawed ≥ 20 metaphase II oocytes (M2s) had a live birth from OC [1]. As expected, we also showed that age at OC and number of M2s thawed were predictive of live birth. Lastly, we demonstrated that patients who underwent two OC cycles had a higher likelihood of live birth than patients who underwent one OC cycle, suggesting that a second OC cycle increases the chance of live birth.
However, it is still unclear whether additional OC cycles independently increase the chance of live birth, or whether the benefit of additional OC cycles can be explained entirely by the increased number of oocytes. Thus, the optimal number of OC cycles and optimal number of oocytes to cryopreserve remain unknown. Patients and physicians often seek guidance from Goldman et al.’s predictive model, which uses data extrapolated from IVF patients with normal ovarian reserve and oocyte donors [9]; however, this model does not account for the number of OC cycles and its correctness is unknown. Another model by Maslow et al. used data from 1799 OC cycles at a single center and estimated live birth rates from previously published data to extrapolate age-based oocyte thresholds for 50%, 60%, and 70% estimated live birth rates [10]. However, the estimated live birth rates were also derived in part from patients with infertility diagnoses and oocyte donors, rather than from planned OC patients alone. Patients yearn for more information about thaw outcomes from actual planned OC patients so they can make informed decisions about the role of OC in achieving their family building goals.
After completing one OC cycle, patients often inquire about: (1) the chance of live birth from their cryopreserved oocytes, and (2) how much additional OC cycles will increase their chance of live birth. However, these questions remain difficult to answer due to the dearth of planned OC outcome data. Therefore, our aim was to examine cumulative live birth rate (CLBR) based on age, total number of M2s thawed, and the total number of OC cycles, with the goal of developing a patient counselling tool.
Materials and Methods
Design
With Institutional Review Board approval (number S13-00389), we performed a retrospective cohort study of all patients who underwent OC followed by autologous oocyte thaw/warming (“thaw” will be used for consistency) at our center prior to December 31, 2023. Transfers from resultant embryos were included if they occurred before the same date.
Subjects
All patients who underwent at least one autologous oocyte thaw at our center during the study period were reviewed. Patients were included if they: (1) had at least one live birth or ongoing pregnancy > 12 weeks from OC, or (2) used all oocytes and euploid and untested embryos from OC. Patients were excluded if: (1) they had remaining cryopreserved oocytes or euploid/untested embryos from OC, but no live birth or ongoing pregnancy > 12 weeks from OC, (2) they had no live birth, but had a transfer of embryos created from OC after December 31, 2023, (3) OC was performed for a medical indication (e.g. fertility preservation prior to gonadotoxic therapy or gender-affirming treatment), due to a natural disaster (Hurricane Sandy), due to lack of sperm, or in combination with embryos, (4) they had a cancer diagnosis or planned to use a gestational carrier, or (5) pregnancy outcome was unknown. Of note, we included patients who underwent OC as part of a research protocol where the intent was to thaw oocytes within the following six months.
Data Collection & Outcomes
Information regarding OC, oocyte thaw, and embryo transfer cycles were obtained from electronic medical records. Collected OC cycle data included: cryopreservation location (our facility, an outside facility, or both); patient age; number of total oocytes and M2 oocytes cryopreserved; and cryopreservation method (slow freezing vs. vitrification vs. both methods). Collected oocyte thaw cycle data included: number of total oocytes and M2 oocytes thawed and surviving thaw; number of usable embryos (defined as: embryos for preimplantation genetic testing (PGT), cryopreservation, or fresh transfer). Collected transfer data included: pregnancy and live birth outcomes. We also determined whether patients had remaining oocytes or euploid/untested embryos from OC in storage at our center or that were transported to another center, donated, or discarded as of December 31, 2023.
The primary outcome was CLBR, and was defined per patient. CLBR included patients who had a live birth or ongoing pregnancy. All ongoing pregnancies were > 12 weeks at data collection. Patients were stratified by age at first OC, total number of M2s thawed, and total number of OC cycles.
OC, Thawing, and Embryo Transfer
Ovarian stimulation protocols were chosen by the treating physician based on the patient’s ovarian reserve and age. From 2004 to 2015, all retrieved M2s and metaphase I oocytes (M1s) were cryopreserved; however, after 2015, M1s were only cryopreserved if less than 15 M2s were obtained from the same OC cycle. Oocytes were cryopreserved with slow freezing or vitrification using previously described techniques [5]. Vitrification was used for all OC cycles performed after July 2011. During our center’s conversion from slow freezing to vitrification, a combination of both OC methods was often used to cryopreserve oocytes from a single retrieval. For this reason, some patients in this study had some oocytes that were slow frozen and some oocytes that were vitrified (from a single OC cycle and/or from multiple different OC cycles).
Oocytes were thawed using previously described techniques [5], and intracytoplasmic sperm injection was used for fertilization. Embryos were cultured until transfer (days 3–7 based on physician instructions), trophectoderm biopsy for PGT, or cryopreservation on days 5–7. In order to be biopsied for PGT, embryos were required to have a Gardner’s score of A or B for the inner cell mass or for the trophectoderm. PGT was performed with array comparative genomic hybridization or next generation sequencing based on what technology was standard in our laboratory at the time of thaw. Endometrial preparation for embryo transfer was achieved using previously described techniques [1]. Typically, embryos with euploid status and/or superior morphology were given preference for transfer.
Statistical Analysis
The Kolmogorov-Smirnoff test was used to assess continuous variables for normality, and these variables were found to be non-parametric. Chi-square tests were used to analyze categorical variables. An alpha error of 0.05 was deemed significant. Results are reported as percentages or medians with interquartile ranges (IQRs).
Lastly, multiple logistic regression was used for modeling and adjustment of covariates (age at first OC, total number of M2s thawed, and total number of OC cycles) to evaluate the outcome of cumulative live birth / ongoing pregnancy. Multiple regression was performed using stepwise addition of parameters that satisfied the Akaike information criterion. 95% confidence intervals were assembled using likelihood intervals (e-fold reduction in maximum likelihood allowing all other parameters to adjust freely). The purpose of this model was to facilitate patient counseling.
Results
A total of 731 patients were included. 527 patients underwent 1 OC cycle, 149 patients underwent 2 OC cycles, 37 patients underwent 3 OC cycles, 10 patients underwent 4 OC cycles, 4 patients underwent 5 OC cycles, 2 patients underwent 6 OC cycles, and 2 patients underwent 8 OC cycles. The median number of OC cycles was 1 and the mean number of OC cycles was 1.4. OC was performed at our facility for 89% of patients, an outside facility for 9% of patients, and both for 2% of patients.
Ninety-six percent of patients (n = 701) did not have a prior infertility diagnosis; 4% (n = 30) of patients underwent OC as part of a research protocol where the intent was to thaw oocytes within the following six months. Patients who underwent OC as part of a research protocol were ≤ 37 years at the time of OC, had normal baseline follicle stimulating hormone levels, and may have had an infertility diagnosis (etiologies: unexplained, polycystic ovarian syndrome, tubal disease).
Table 1 shows the age distribution of our cohort at the time of first OC. The median age at first OC was 38y (IQR: 36—39). The median number of M2s cryopreserved in the first OC cycle was 10 (IQR: 6—16), and the median total number of M2s cryopreserved was 13 (IQR: 8—20). The cryopreservation method was vitrification alone for 78% of patients, slow freezing alone for 4% of patients, a combination of both vitrification and slow freezing for 18% of patients, and unknown for < 1% of patients.
The median age at first thaw was 42 years (IQR: 40 – 44), and the median time between first OC and first thaw was 4.6 years (IQR: 3.0 – 6.1 years; maximum: 13.2 years). The median total number of M2s thawed was 12 (IQR: 8 – 18.5) and the median total number M2s that survived thaw was 10 (IQR: 5—15). Patients had a median of 3 usable embryos (IQR 1 – 5) from OC. Among patients with ≥ 1 useable embryo, 73% (n = 449/618) used PGT-A to test ≥ 1 embryo. The vast majority of embryo transfers (92%) were performed on day 5 or 6; however, 4% were performed on day 3 or 4 and 4% were performed on day 7. The CLBR among all patients was 43% (n = 313/731).
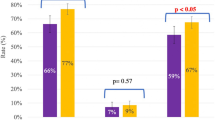
CLBRs did not differ based on whether patients underwent 1, 2, or ≥ 3 OC cycles (p = 0.20). The CLBR was 41% (n = 215/527) among patients who underwent 1 OC cycle, 48% (n = 71/149) among patients who underwent 2 OC cycles, and 49% (n = 27/55) among patients who underwent ≥ 3 OC cycles.
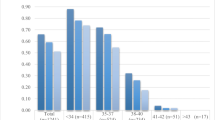
CLBR differed among patients of different ages (p < 0.01) and among patients who thawed different numbers of M2s (p < 0.01). Figure 1. shows CLBR by age, and Fig. 2 shows CLBR by total number of M2s thawed. Table 2 shows CLBR when patients were stratified by age at first cryopreservation and total number of M2s thawed.
Multiple logistic regression shows that age at first OC (B = -0.14; 95% confidence interval [CI] = -0.19 to -0.10), and total number of M2s thawed (B = 0.07; 95% CI = 0.06 to 0.08) independently predicted CLBR. The number of OC cycles was not included in the multiple regression model since it was not a significant predictor of live birth (failed to exceed the Akaike information criterion and its coefficient was not significantly different than zero when included in the model). The variance inflation factors were as follows: age at first OC = 1.04, total number of M2s thawed = 1.23, number of OC cycles = 1.19. Based on these values, we can conclude that there is not sufficient multicollinearity to confound the use of these three parameters.
Figure 3 shows CLBRs from our multiple logistic regression model. As expected, this model shows that CLBR is influenced by the age at OC and the number of M2s thawed.
In total, we report 313 patients with ≥ 1 live birth or ongoing pregnancy > 12 weeks from OC. These patients have a total of 370 babies or fetuses > 12 weeks. 258 patients had one live birth or fetus > 12 weeks, 50 patients had two live births or fetuses > 12 weeks (31 patients with two singletons, 19 patients with twins), and 4 patients had three live births (1 patient with 3 singletons, 2 patients with twins followed by a singleton, 1 patient with triplets) from OC. Among all patients, 26% (n = 188/731) have remaining cryopreserved oocytes and/or euploid/untested embryos from OC. Among those with one live birth or fetus > 12 weeks from OC, 57% (n = 148/258) have remaining cryopreserved oocytes and/or euploid/untested embryos from OC.
Discussion
As use of OC increases, outcome data must be published so patients can make educated decisions about how to best employ this technology to preserve their fertility. Patients must be counselled that younger age at OC and more mature oocytes improve cumulative live birth rates. However, additional OC cycles from different months do not independently improve cumulative live birth rates. The benefit of additional OC cycles can be explained by the increased number of oocytes. Our results provide more information about the CLBR from OC based on age at OC and number of cryopreserved oocytes. This data can help patients decide how many oocytes to cryopreserve.
We provide a CLBR per patient, which is the most useful parameter when counselling OC patients. Our model, which predicts CLBR based on age at OC and total number of M2s thawed can help physicians provide personalized patient counselling. This information not only arms patients with realistic expectations, but can also help them decide whether to pursue additional OC cycles to obtain more oocytes. For example, a 34 year-old patient with 10 M2s from her first OC cycle can be counseled that cryopreserving 10 additional M2s will improve CLBR from approximately 49% to approximately 66%.
The optimal number of oocytes to cryopreserve varies for each patient. This number is not only influenced by the age at OC, but also by the patient’s ovarian reserve, desired likelihood of success, family building goals, and financial situation. Counseling must be individualized, but the predicted CLBRs from our models can help patients decide whether they would benefit from additional oocytes from additional OC cycles. Younger patients with a high number of oocytes from their first OC cycle may decide that the risks and cost of additional OC cycles outweigh the benefits.
Due to the time delay between OC and oocyte thaw, 38% of patients in this cohort underwent their first OC cycle between 2004 and 2012. OC was experimental during this time, and was not covered by employers or insurance. Due to the experimental nature and high cost of OC, patients who obtained a reasonable number of oocytes during their first OC cycle rarely pursued additional OC cycles. By contrast, patients who obtained a small number of oocytes during their first OC cycle often chose to pursue additional OC cycles to increase their chance of live birth. Now that OC is no longer considered experimental and is sometimes covered by employers or insurance, more patients with a reasonable number of oocytes from their first OC cycle are electing to pursue additional OC cycles.
This study does not provide information about the chance of achieving a second live birth from OC because most of our patients with one live birth from OC have not yet returned to use their remaining embryos. However, we report 50 patients with ≥ 2 children from OC, and the fact that 57% of patients had remaining cryopreserved oocytes and euploid/untested embryos from OC after one live birth suggests that many patients can build larger families from OC.
It is also important to note that our study includes oocytes that were slow-frozen in addition to those that were vitrified. Although our center now exclusively uses vitrification to cryopreserve oocytes, previous data from our center demonstrates that our slow-frozen oocytes performed very well [1, 11]. At our center, the decision to use vitrification alone was primarily due to laboratory efficiency, rather than improved outcomes with this cryopreservation method.
One major strength of this study is that it uses real OC data, rather than data based on IVF patients and oocyte donors. Our CLBRs for younger patients are lower than the CLBRs predicted by both Fox and Goldman.’s [12] and Maslow et al.’s [10] models. For example, Fox and Goldman’s model predicts that patients who undergo OC at age 34 and cryopreserve 6, 12, 18, and 24 M2s have CLBRs of 50%, 75%, 88%, and 94% respectively, while our model predicts respective CLBRs of 42%, 52%, 62%, and 71% for these patients. Similarly, Maslow et. al’s model predicts that women aged < 35 years have a 70% estimated live birth rate when 10 M2s are obtained from their first retrieval and a 80% predicted live birth rate when 17 M2s are obtained from their first retrieval, while our model predicts lower live birth rates for women, especially as they approach 35 years. Therefore, we suggest that OC models that rely on extrapolated data from IVF patients with normal ovarian reserve and oocyte donors be used with caution because they likely overestimate CLBRs for younger patients. We recommend using data from real OC patients for patient counseling.
This study is limited because Anti-Müllerian Hormone (AMH), Body Mass Index (BMI), and race/ethnicity were not included in our analysis and may impact the number of oocytes retrieved and live birth rates. This study is also limited by the relatively small number of OC patients who have returned for thaw. Many OC patients do not return to use their cryopreserved oocytes, and those who do return to use their cryopreserved oocytes tend to return several years later [13, 14]. Moreover, the majority of patients (72%) in this study only underwent 1 OC cycle. Larger studies are required to corroborate our findings about the effect of cycle number and to more precisely detect differences in CLBR based on age at OC and number of M2s. Moreover, this study includes patients who underwent oocyte thaw at a single high-volume urban university-affiliated institution and may not be generalizable to other OC patients. Further studies are needed from a variety center types and locations.
In conclusion, the number of cryopreserved M2s predicts CLBR, even at young ages. However, additional OC cycles do not independently improve cumulative live birth rates. This study is the first report of oocyte thaw outcomes to examine the influence of OC cycle number on CLBR, and suggests that additional OC cycles do not increase CLBR beyond increasing oocyte quantity. Our results can help OC patients estimate their CLBR and decide whether to pursue additional OC cycles to obtain more oocytes.
Data Availability
The data included in this study is not publically available.
References
Cascante SD, Blakemore JK, DeVore S, Hodes-Wertz B, Fino ME, Berkeley AS, et al. Fifteen years of autologous oocyte thaw outcomes from a large university-based fertility center. Fertil Steril. 2022;118:158–66.
Noyes N, Porcu E, Borini A. Over 900 oocyte cryopreservation babies born with no apparent increase in congenital anomalies. Reprod Biomed Online. 2009;18:769–76.
Grifo JA, Noyes N. Delivery rate using cryopreserved oocytes is comparable to conventional in vitro fertilization using fresh oocytes: potential fertility preservation for female cancer patients. Fertil Steril. 2010;93:391–6.
Hodes-Wertz B, Noyes N, Mullin C, McCaffrey C, Grifo JA. Retrospective analysis of outcomes following transfer of previously cryopreserved oocytes, pronuclear zygotes and supernumerary blastocysts. Reprod Biomed Online. 2011;23:118–23.
Goldman KN, Noyes NL, Knopman JM, McCaffrey C, Grifo JA. Oocyte efficiency: does live birth rate differ when analyzing cryopreserved and fresh oocytes on a per-oocyte basis? Fertil Steril. 2013;100:712–7.
Trokoudes KM, Pavlides C, Zhang X. Comparison outcome of fresh and vitrified donor oocytes in an egg-sharing donation program. Fertil Steril. 2011;95:1996–2000.
Cobo A, Meseguer M, Remohí J, Pellicer A. Use of cryo-banked oocytes in an ovum donation programme: a prospective, randomized, controlled, clinical trial. Hum Reprod (Oxford, England). 2010;25:2239–46.
Levi-Setti PE, Borini A, Patrizio P, Bolli S, Vigiliano V, De Luca R, et al. ART results with frozen oocytes: data from the Italian ART registry (2005–2013). J Assist Reprod Genet. 2016;33:123–8.
Goldman RH, Racowsky C, Farland LV, Munne S, Ribustello L, Fox JH. Predicting the likelihood of live birth for elective oocyte cryopreservation: a counseling tool for physicians and patients. Hum Reprod (Oxford, England). 2017;32:853–9.
Maslow BL, Guarnaccia MM, Ramirez L, Klein JU. Likelihood of achieving a 50%, 60%, or 70% estimated live birth rate threshold with 1 or 2 cycles of planned oocyte cryopreservation. J Assist Reprod Genet. 2020;37:1637–43.
Noyes N, Knopman J, Labella P, McCaffrey C, Clark-Williams M, Grifo J. Oocyte cryopreservation outcomes including pre-cryopreservation and post-thaw meiotic spindle evaluation following slow cooling and vitrification of human oocytes. Fertil Steril. 2010;94:2078–82.
Fox J, Goldman R. BWH Egg Freezing Counseling Tool (EFCT), MDCalc. www.mdcalc.com/calc/3937/bwh-egg-freezing-counseling-tool-efct. Accessed 26 June 2024.
Blakemore JK, Grifo JA, DeVore SM, Hodes-Wertz B, Berkeley AS. Planned oocyte cryopreservation-10-15-year follow-up: return rates and cycle outcomes. Fertil Steril. 2021;115:1511–20.
Leung AQ, Baker K, Vaughan D, Shah JS, Korkidakis A, Ryley DA, et al. Clinical outcomes and utilization from over a decade of planned oocyte cryopreservation. Reprod Biomed Online. 2021;43:671–9.
Acknowledgements
The authors gratefully acknowledge the patients included in this study as well as the entire New York University Langone Fertility Center. We also acknowledge and thank the Carolyn and Malcom Wiener Foundation for their generous support, which funded much of the foundational scientific studies that led to our clinically successful oocyte cryopreservation program. Lastly, we acknowledge Dr. David McCulloh who assisted with the multiple logistic regression model.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interests
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cascante, S.D., Grifo, J.A., Licciardi, F. et al. The effects of age, mature oocyte number, and cycle number on cumulative live birth rates after planned oocyte cryopreservation. J Assist Reprod Genet (2024). https://doi.org/10.1007/s10815-024-03175-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10815-024-03175-w