Abstract
Purpose
This study aimed to identify the genetic causes of male infertility and primary ciliary dyskinesia (PCD)/PCD-like phenotypes in three unrelated Han Chinese families.
Methods
We conducted whole-exome sequencing of three patients with male infertility and PCD/PCD-like phenotypes from three unrelated Chinese families. Ultrastructural and immunostaining analyses of patient spermatozoa and respiratory cilia and in vitro analyses were performed to analyze the effects of SPEF2 variants. Intracytoplasmic sperm injection (ICSI) was administered to three affected patients.
Results
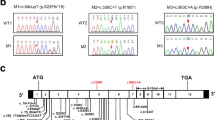
We identified four novel SPEF2 variants, including one novel homozygous splicing site variant [NC_000005.10(NM_024867.4): c.4447 + 1G > A] of the SPEF2 gene in family 1, novel compound heterozygous nonsense variants [NC_000005.10(NM_024867.4): c.1339C > T (p.R447*) and NC_000005.10(NM_024867.4): c.1645G > T (p.E549*)] in family 2, and one novel homozygous missense variant [NC_000005.10(NM_024867.4): c.2524G > A (p.D842N)] in family 3. All the patients presented with male infertility and PCD/likely PCD. All variants were present at very low levels in public databases, predicted to be deleterious in silico prediction tools, and were further confirmed deleterious by in vitro analyses. Ultrastructural analyses of the spermatozoa of the patients revealed the absence of the central pair complex in the sperm flagella. Immunostaining of the spermatozoa and respiratory cilia of the patients validated the pathogenicity of the SPEF2 variants. All patients carrying SPEF2 variants underwent one ICSI cycle and delivered healthy infants.
Conclusion
Our study reported four novel pathogenic variants of SPEF2 in three male patients with infertility and PCD/PCD-like phenotypes, which not only extend the spectrum of SPEF2 mutations but also provide information for genetic counseling and treatment of such conditions.




Similar content being viewed by others
Data availability
The data of this study are included in the article and Supplementary Material. Further inquiries can be acquired from the corresponding author upon reasonable request.
References
Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, De Mouzon J, Sokol R, et al. The international glossary on infertility and fertility care, 2017. Hum Reprod. 2017;32(9):1786–801. https://doi.org/10.1093/humrep/dex234.
Sharlip ID, Jarow JP, Belker AM, Lipshultz LI, Sigman M, Thomas AJ, et al. Best practice policies for male infertility. Fertil Steril. 2002;77(5):873–82. https://doi.org/10.1016/s0015-0282(02)03105-9.
Vander Borght M, Wyns C Fertility and infertility: definition and epidemiology. Clin Biochem. 2018;622–10. https://doi.org/10.1016/j.clinbiochem.2018.03.012.
Cooper TG, Noonan E, Von Eckardstein S, Auger J, Baker HW, Behre HM, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16(3):231–45. https://doi.org/10.1093/humupd/dmp048.
He X, Liu C, Yang X, Lv M, Ni X, Li Q, et al. Bi-allelic loss-of-function variants in CFAP58 cause flagellar axoneme and mitochondrial sheath defects and asthenoteratozoospermia in humans and mice. Am J Hum Genet. 2020;107(3):514–26. https://doi.org/10.1016/j.ajhg.2020.07.010.
Tu C, Cong J, Zhang Q, He X, Zheng R, Yang X, et al. Bi-allelic mutations of DNAH10 cause primary male infertility with asthenoteratozoospermia in humans and mice. Am J Hum Genet. 2021;108(8):1466–77. https://doi.org/10.1016/j.ajhg.2021.06.010.
Paff T, Omran H, Nielsen KG, Haarman EG. Current and future treatments in primary ciliary dyskinesia. Int J Mol Sci. 2021;22(18). https://doi.org/10.3390/ijms22189834.
Sironen A, Shoemark A, Patel M, Loebinger MR, Mitchison HM. Sperm defects in primary ciliary dyskinesia and related causes of male infertility. Cell Mol Life Sci. 2020;77(11):2029–48. https://doi.org/10.1007/s00018-019-03389-7.
Jayasena CN, Sironen A. diagnostics and management of male infertility in primary ciliary dyskinesia. Diagnostics (Basel). 2021;11(9). https://doi.org/10.3390/diagnostics11091550.
Newman L, Chopra J, Dossett C, Shepherd E, Bercusson A, Carroll M, et al. The impact of primary ciliary dyskinesia on female and male fertility: a narrative review. Hum Reprod Update. 2023;29(3):347–67. https://doi.org/10.1093/humupd/dmad003.
Li Y, Li Y, Wang Y, Meng L, Tan C, Du J, et al. Identification of novel biallelic LRRC6 variants in male Chinese patients with primary ciliary dyskinesia and infertility. J Assist Reprod Genet. 2023;40(1):41–51. https://doi.org/10.1007/s10815-022-02681-z.
Blanchon S, Legendre M, Copin B, Duquesnoy P, Montantin G, Kott E, et al. Delineation of CCDC39/CCDC40 mutation spectrum and associated phenotypes in primary ciliary dyskinesia. J Med Genet. 2012;49(6):410–6. https://doi.org/10.1136/jmedgenet-2012-100867.
Li Y, Wang WL, Tu CF, Meng LL, Hu TY, Du J, et al. A novel homozygous frameshift mutation in MNS1 associated with severe oligoasthenoteratozoospermia in humans. Asian J Androl. 2021;23(2):197–204. https://doi.org/10.4103/aja.aja_56_20.
Leslie JS, Rawlins LE, Chioza BA, Olubodun OR, Salter CG, Fasham J, et al. MNS1 variant associated with situs inversus and male infertility. Eur J Hum Genet. 2020;28(1):50–5. https://doi.org/10.1038/s41431-019-0489-z.
Ta-Shma A, Hjeij R, Perles Z, Dougherty GW, Abu Zahira I, Letteboer SJF, et al. Homozygous loss-of-function mutations in MNS1 cause laterality defects and likely male infertility. PLoS Genet. 2018;14(8):e1007602. https://doi.org/10.1371/journal.pgen.1007602.
Zuccarello D, Ferlin A, Cazzadore C, Pepe A, Garolla A, Moretti A, et al. Mutations in dynein genes in patients affected by isolated non-syndromic asthenozoospermia. Hum Reprod. 2008;23(8):1957–62. https://doi.org/10.1093/humrep/den193.
Zhu D, Zhang H, Wang R, Liu X, Jiang Y, Feng T, et al. Association of DNAH11 gene polymorphisms with asthenozoospermia in Northeast Chinese patients. Biosci Rep. 2019;39(6). 10.1042/BSR20181450.
Knowles MR, Leigh MW, Carson JL, Davis SD, Dell SD, Ferkol TW, et al. Mutations of DNAH11 in patients with primary ciliary dyskinesia with normal ciliary ultrastructure. Thorax. 2012;67(5):433–41. https://doi.org/10.1136/thoraxjnl-2011-200301.
Ostrowski LE, Andrews K, Potdar P, Matsuura H, Jetten A, Nettesheim P. Cloning and characterization of KPL2, a novel gene induced during ciliogenesis of tracheal epithelial cells. Am J Respir Cell Mol Biol. 1999;20(4):675–83. https://doi.org/10.1165/ajrcmb.20.4.3496.
Sironen A, Thomsen B, Andersson M, Ahola V, Vilkki J. An intronic insertion in KPL2 results in aberrant splicing and causes the immotile short-tail sperm defect in the pig. Proc Natl Acad Sci U S A. 2006;103(13):5006–11. https://doi.org/10.1073/pnas.0506318103.
Sironen A, Hansen J, Thomsen B, Andersson M, Vilkki J, Toppari J, et al. Expression of SPEF2 during mouse spermatogenesis and identification of IFT20 as an interacting protein. Biol Reprod. 2010;82(3):580–90. https://doi.org/10.1095/biolreprod.108.074971.
Cindric S, Dougherty GW, Olbrich H, Hjeij R, Loges NT, Amirav I, et al. SPEF2- and HYDIN-mutant cilia lack the central pair-associated protein SPEF2, aiding primary ciliary dyskinesia diagnostics. Am J Respir Cell Mol Biol. 2020;62(3):382–96. https://doi.org/10.1165/rcmb.2019-0086OC.
Tu C, Nie H, Meng L, Wang W, Li H, Yuan S, et al. Novel mutations in SPEF2 causing different defects between flagella and cilia bridge: the phenotypic link between MMAF and PCD. Hum Genet. 2020;139(2):257–71. https://doi.org/10.1007/s00439-020-02110-0.
Liu C, Lv M, He X, Zhu Y, Amiri-Yekta A, Li W, et al. Homozygous mutations in SPEF2 induce multiple morphological abnormalities of the sperm flagella and male infertility. J Med Genet. 2020;57(1):31–7. https://doi.org/10.1136/jmedgenet-2019-106011.
Sha Y, Liu W, Wei X, Zhu X, Luo X, Liang L, et al. Biallelic mutations in sperm flagellum 2 cause human multiple morphological abnormalities of the sperm flagella (MMAF) phenotype. Clin Genet. 2019;96(5):385–93. https://doi.org/10.1111/cge.13602.
Guo F, Yang B, Ju ZH, Wang XG, Qi C, Zhang Y, et al. Alternative splicing, promoter methylation, and functional SNPs of sperm flagella 2 gene in testis and mature spermatozoa of Holstein bulls. Reproduction. 2014;147(2):241–52. https://doi.org/10.1530/REP-13-0343.
Sironen A, Kotaja N, Mulhern H, Wyatt TA, Sisson JH, Pavlik JA, et al. Loss of SPEF2 function in mice results in spermatogenesis defects and primary ciliary dyskinesia. Biol Reprod. 2011;85(4):690–701. https://doi.org/10.1095/biolreprod.111.091132.
Mckenzie CW, Lee L. Genetic interaction between central pair apparatus genes CFAP221, CFAP54, and SPEF2 in mouse models of primary ciliary dyskinesia. Sci Rep. 2020;10(1):12337. https://doi.org/10.1038/s41598-020-69359-3.
Liu W, Sha Y, Li Y, Mei L, Lin S, Huang X, et al. Loss-of-function mutations in SPEF2 cause multiple morphological abnormalities of the sperm flagella (MMAF). J Med Genet. 2019;56(10):678–84. https://doi.org/10.1136/jmedgenet-2018-105952.
Mori M, Kido T, Sakamoto N, Ozasa M, Kido K, Noguchi Y, et al. Novel SPEF2 variant in a Japanese patient with primary ciliary dyskinesia: a case report and literature review. J Clin Med. 2022;12(1). https://doi.org/10.3390/jcm12010317.
Lucas JS, Barbato A, Collins SA, Goutaki M, Behan L, Caudri D, et al. European Respiratory Society guidelines for the diagnosis of primary ciliary dyskinesia. Eur Respir J. 2017;49(1). https://doi.org/10.1183/13993003.01090-2016.
Leigh MW, Hazucha MJ, Chawla KK, Baker BR, Shapiro AJ, Brown DE, et al. Standardizing nasal nitric oxide measurement as a test for primary ciliary dyskinesia. Ann Am Thorac Soc. 2013;10(6):574–81. https://doi.org/10.1513/AnnalsATS.201305-110OC.
Tan YQ, Tu C, Meng L, Yuan S, Sjaarda C, Luo A, et al. Loss-of-function mutations in TDRD7 lead to a rare novel syndrome combining congenital cataract and nonobstructive azoospermia in humans. Genet Med. 2019;21(5):1209–17. https://doi.org/10.1038/gim.2017.130.
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–24. https://doi.org/10.1038/gim.2015.30.
Gu YF, Zhou QW, Zhang SP, Lu CF, Gong F, Shi Y, et al. The clinical and neonatal outcomes after stimulation of immotile spermatozoa using SperMagic medium. Andrologia. 2018;50(7):e13056. https://doi.org/10.1111/and.13056.
Acknowledgements
The authors wish to sincerely thank all the families and individuals who participated in this study. The support from the technical platform and clinicians involved at the Genetic Hospital of CITIC-Xiangya is gratefully acknowledged.
Funding
The authors wish to sincerely thank all the families and individuals who participated in this study. This work was supported by the National Key Research and Developmental Program of China (2022YFC2702604 to Y.-Q.T.), the National Natural Science Foundation of China (82171608 to Y.-Q.T., 82101961 to CF.T.), the outstanding Youth Foundation of Hunan Provincial Natural Science Foundation of China (2023JJ20080 to CF.T.), and the Hunan Provincial Natural Science Foundation of China (2023JJ30731 to Y.-Q.T., 2022JJ30772 to J. D.).
Author information
Authors and Affiliations
Contributions
Juan Du, Haichun Guo, and Longxiang Wu designed the study. Lanlan Meng and Chen Tan performed the variant analysis. Wenqing Lu, Yong Li, Chaofeng Tu, Yuying Song, and Hongchuan Nie carried out the evaluation of the pathogenicity of variations and spermatozoa functional analyses. Yue-Qiu Tan, Qianjun Zhang, Guangxiu Lu, and Ge Lin worked on the clinical study. Wenqing Lu and Yong Li wrote the paper. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Haichun Guo, Longxiang Wu, and Juan Du cosupervised the study and should be considered shared last authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lu, W., Li, Y., Meng, L. et al. Novel SPEF2 variants cause male infertility and likely primary ciliary dyskinesia. J Assist Reprod Genet (2024). https://doi.org/10.1007/s10815-024-03106-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10815-024-03106-9